Cyclobutadieneiron tricarbonyl
Cyclobutadieneiron tricarbonyl or (C4H4)Fe(CO)3 is an organoiron compound with the formula Fe(C4H4)(CO)3. It is a yellow solid that is soluble in organic solvents. It has been used in organic chemistry as a precursor for cyclobutadiene, which is an elusive species in the free state.[1]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| |
| Properties | |
| (C4H4)Fe(CO)3 | |
| Appearance | yellow solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and structure
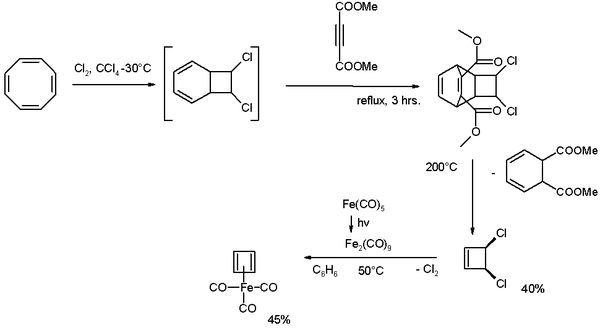
It was first prepared in 1965 by Rowland Pettit starting from cyclooctatetraene:[2][3][4]
Cyclooctatetraene is chlorinated to the [4.2.0]-bicyclic compound which reacts further with the alkyne dimethyl acetylenedicarboxylate in a Diels-Alder reaction followed by a reverse-DA reaction by pyrolysis at 200 °C releasing cis-dichlorocyclobutene. This compound reacts with di-iron nonacarbonyl (obtained from photolysis of iron pentacarbonyl) to give cyclobutadieneiron tricarbonyl.
The compound is a half sandwich complex. The C-C distances are 1.426 Å.[5]
Properties
Oxidative decomplexation of cyclobutadiene is achieved by treating the tricarbonyl complex with ceric ammonium nitrate. The released cyclobutadiene is trapped with a quinone, which functions as a dienophile.[6]
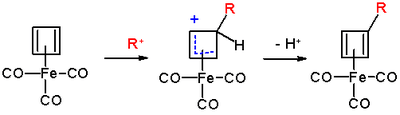
Cyclobutadieneiron tricarbonyl displays aromaticity as evidenced by some of its reactions, which can be classified as electrophilic aromatic substitution:[7]
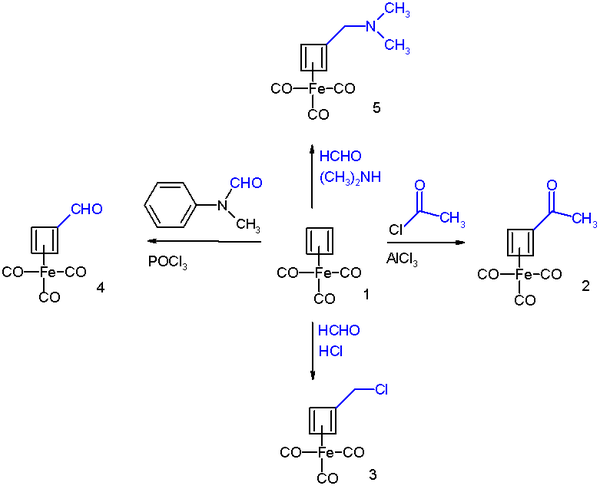
It undergoes Friedel-Crafts acylation with acetyl chloride and aluminium chloride to give the acyl derivative 2, with formaldehyde and hydrochloric acid to the chloromethyl derivative 3, in a Vilsmeier-Haack reaction with N-methylformanilide and phosphorus oxychloride to the formyl 4, and in a Mannich reaction to amine derivative 5.
The reaction mechanism is identical to that of EAS:
Related compounds
Several years before Petit's work, (C4Ph4)Fe(CO)3 had been prepared from the reaction of iron carbonyl and diphenylacetylene.[8]
(Butadiene)iron tricarbonyl is isoelectronic with cyclobutadieneiron tricarbonyl.
References
- D. Seyferth "(Cyclobutadiene)iron Tricarbonyl - A Case of Theory before Experiment" Organometallics 2003, volume 22, 2-20.
- Cyclobutadiene- and Benzocyclobutadiene-Iron Tricarbonyl Complexes G. F. Emerson, L. Watts, R. Pettit; J. Am. Chem. Soc.; 1965; 87(1); 131-133. First Page
- Cis-dichlorocyclobutene , Organic Syntheses, Coll. Vol. 6, p.422 (1988); Vol. 50, p.36 (1970) Article Archived 2011-06-05 at the Wayback Machine.
- Iron, tricarbonyl (η4-1,3-cyclobutadiene)- R. Pettit and J. Henery Organic Syntheses, Coll. Vol. 6, p.310 (1988); Vol. 50, p.21 (1970) Link
- P. D. Harvey; W. P. Schaefer; H. B. Gray; D. F. R. Gilson; I. S. Butler (1988). "Structure of tricarbonyl(η4-cyclobutadienyl)iron(0) at −45 °C". Inorg. Chem. 27 (1): 57–59. doi:10.1021/ic00274a013.
- L. Brener, J. S. Mckennis, R. Pettit (1976). "Cyclobutadiene In Synthesis: endo-Tricyclo[4.4.0.02,5]deca-3,8-diene-7,10-dione". Org. Synth. 55: 43. doi:10.15227/orgsyn.055.0043.CS1 maint: uses authors parameter (link)
- Cyclobutadieneiron Tricarbonyl. A New Aromatic System J. D. Fitzpatrick, L. Watts, G. F. Emerson, R. Pettit J. Am. Chem. Soc.; 1965, vol. 87, 3254-3255 Abstract
- R. P. Dodge, V. Schomaker, "Crystal Structure of Tetraphenylcyclobutadiene Iron Tricarbonyl", Nature 1960, vol. 186, 798-799.doi:10.1038/186798b0