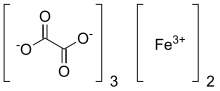
Ferric oxalate
Ferric oxalate, also known as iron(III) oxalate, is a chemical compound composed of ferric ions and oxalate ligands; it may also be regarded as the ferric salt of oxalic acid. The anhydrous material is pale yellow; however, it may be hydrated to form several hydrates, such as potassium ferrioxalate, or Fe
2(C
2O
4)
3 • 6H2O, which is bright green in colour.
 | |
| Names | |
|---|---|
| Systematic IUPAC name
iron(3+) ethanedioate (2:3) | |
| Other names
Iron(III) oxalate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.019.047 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6Fe2O12 | |
| Molar mass | 375.747 g/mol |
| Appearance | Pale yellow solid (anhydrous) Lime green solid (hexahydrate) |
| Odor | odorless |
| slightly soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
Tetrahydrate
2(C
2O
4)
3·4H2O
The crystal structure of the tetrahydrate Fe
2(C
2O
4)
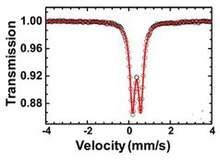
3 • 4H2O was determined in 2015. It has a triclinic unit cell containing two iron atoms. Each iron atom has octahedral coordination bonds to the oxygen atoms of three oxalate molecules and one water molecule. Two of those three oxalates, lying in approximately perpendicular planes, are tetradentate, and connect the iron atoms into zigzag chains. The third oxalate molecule is bidentate, and connects iron atoms of adjacent chains, creating an open-layered structure. Half of the water molecules lie, unbound, between those chains. Mössbauer spectrum of Fe
2(C
2O
4)
3 • 4H2O indicates that iron is present in a unique environment with an isomer shift of 0.38 mm/s and a quadrupole splitting of 0.40 mm/s, suggesting a high spin Fe3+ in octahedral coordination.[1]
Uses
Dentistry
Like many oxalates, ferric oxalate has been investigated as a short term treatment for dentin hypersensitivity.[2] It is used in certain toothpaste formulations; however, its effectiveness has been questioned.[3]
Photography
Ferric oxalate is used as the light-sensitive element in the Kallitype photographic printing process; and the platinotype process Platinum/Palladium Printing.
Batteries
Ferric oxalate tetrahydrate has been investigated as a possible cheap material for the positive electrode of lithium-iron batteries. It can intercalate lithium ions at an average potential of 3.35 V, and has shown a sustainable capacity of 98 mAh/g.[1]
See also
A number of other iron oxalates are known:-
References
- Ahouari, Hania; Rousse, Gwenaëlle; Rodríguez-Carvajal, Juan; Sougrati, Moulay-Tahar; Saubanère, Matthieu; Courty, Matthieu; Recham, Nadir; Tarascon, Jean-Marie (2015). "Unraveling the Structure of Iron(III) Oxalate Tetrahydrate and Its Reversible Li Insertion Capability". Chemistry of Materials. 27 (5): 1631–1639. doi:10.1021/cm5043149.
- Gillam, D. G.; Newman, H. N.; Davies, E. H.; Bulman, J. S.; Troullos, E. S.; Curro, F. A. (2004). "Clinical evaluation of ferric oxalate in relieving dentine hypersensitivity". Journal of Oral Rehabilitation. 31 (3): 245–250. doi:10.1046/j.0305-182X.2003.01230.x.
- Cunha-Cruz, J.; Stout, J. R.; Heaton, L. J.; Wataha, J. C. (29 December 2010). "Dentin Hypersensitivity and Oxalates: a Systematic Review". Journal of Dental Research. 90 (3): 304–310. doi:10.1177/0022034510389179. PMC 3144108. PMID 21191127.