Sulfur tetrachloride
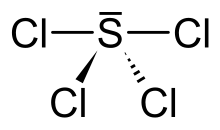
Sulfur tetrachloride is an inorganic compound with chemical formula SCl4. It has only been obtained as an unstable pale yellow solid. The corresponding SF4 is a stable, useful reagent.
 | |
| Names | |
|---|---|
| IUPAC name
Sulfur(IV) chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.149.178 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| SCl4 | |
| Molar mass | 173.87 |
| Appearance | White powder |
| Melting point | −31 °C (−24 °F; 242 K) |
| Boiling point | −20 °C (−4 °F; 253 K) (decomposes) |
| soluble in water | |
| Hazards | |
| R-phrases (outdated) | R14, R34, R50 |
| S-phrases (outdated) | (S1/2), S26, S45, S61 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and structure
It is obtained by treating sulfur dichloride with chlorine at 193 K:
-
(1)
It melts with simultaneous decomposition above −20 °C.[1]
Its solid structure is uncertain. It is probably the salt SCl3+Cl−, since related salts are known with noncoordinating anions.[2][3] In contrast to this tetrachloride, SF4 is a neutral molecule.[4]
Reactions
It decomposes above −30 °C (242 K) to sulfur dichloride and chlorine.
-
(2)
It hydrolyzes readily:
-
(3)
Sulfur tetrachloride reacts with water, producing hydrogen chloride and sulfur dioxide through the hydrolysis process. Thionyl chloride is an implied intermediate.[5]
-
(4)
- Oxidized by nitric acid:
-
(5)
References
- Georg Brauer: Handbuch der Präparativen Anorganischen Chemie. (in German)
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Christian, Beverly H.; Collins, Michael J.; Gillespie, Ronald J.; Sawyer, Jeffery F. "Preparations, Raman spectra, and crystal structures of (SCl3)(SbCl6), (SeCl3)(SbCl6), (SBr1.2Cl1.8)(SbCl6), (TeCl3)(AlCl4) (triclinic modification), (TeCl3)(SbF6), (TeCl3)(AsF6), and (TeF3)2(SO4)" Inorganic Chemistry 1986, volume 25, 777-88. doi:10.1021/ic00226a012
- Goettel, J. T., Kostiuk, N. and Gerken, M. (2013), The Solid-State Structure of SF4: The Final Piece of the Puzzle . Angew. Chem. Int. Ed., 52: 8037–8040. doi:10.1002/anie.201302917
- Holleman-Wiberg, Lehrbuch der Anorganischen Chemie, 101. Auflage, de Gruyter Verlag 1995 ISBN 3-11-012641-9 (in German)