Omeprazole
Omeprazole, sold under the brand names Prilosec and Losec among others, is a medication used in the treatment of gastroesophageal reflux disease (GERD), peptic ulcer disease, and Zollinger–Ellison syndrome.[1] It is also used to prevent upper gastrointestinal bleeding in people who are at high risk.[1] Omeprazole is a proton-pump inhibitor (PPI) and its effectiveness is similar to other PPIs.[7] It can be taken by mouth or by injection into a vein.[1][8]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /oʊˈmɛprəzoʊl/ |
| Trade names | Losec, Prilosec, Zegerid, others[1][2] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693050 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth, IV |
| Drug class | Proton-pump inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 35–76%[4][5] |
| Protein binding | 95% |
| Metabolism | Hepatic (CYP2C19, CYP3A4) |
| Elimination half-life | 1–1.2 hours |
| Excretion | 80% (urine) 20% (bile via feces) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.122.967 |
| Chemical and physical data | |
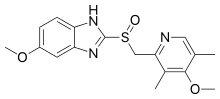
| Formula | C17H19N3O3S |
| Molar mass | 345.42 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Density | 1.4±0.1[6] g/cm3 |
| Melting point | 156 °C (313 °F) |
| |
| |
| (verify) | |
Common side effects include nausea, vomiting, headaches, abdominal pain, and increased intestinal gas.[1][9] Serious side effects may include Clostridium difficile colitis, an increased risk of pneumonia, an increased risk of bone fractures, and the potential of masking stomach cancer.[1] It is unclear if it is safe for use in pregnancy.[1] It works by blocking the release of stomach acid.[1]
Omeprazole was patented in 1978, and approved for medical use in 1988.[10] It is on the World Health Organization's List of Essential Medicines.[11] It is available as a generic medication.[1] In 2017, it was the seventh most commonly prescribed medication in the United States, with more than 58 million prescriptions.[12][13] It is also available without a prescription in the United States.[14]
Medical uses
Omeprazole can be used in the treatment of gastroesophageal reflux disease (GERD), peptic ulcers, erosive esophagitis, Zollinger-Ellison syndrome, and eosinophilic esophagitis.[15][1]
Peptic ulcers
Peptic ulcers may be treated with omeprazole. Treatment of a Helicobacter pylori infection can be completed by taking a triple therapy combination of omeprazole, amoxicillin, and clarithromycin for 7–14 days.[16] Amoxicillin may be replaced with metronidazole in patients who are allergic to penicillin.[17]
Adverse effects
Adverse effects occurring in at least 1% of people include:[18]
- Central nervous system: headache (7%), dizziness (2%)
- Respiratory: upper respiratory tract infection (2%), cough (1%)
- Gastrointestinal: abdominal pain (5%), diarrhea (4%), nausea (4%), vomiting (3%), flatulence (3%), acid regurgitation (2%), constipation (2%)
- Neuromuscular and skeletal: back pain (1%), weakness (1%)
- Dermatologic: rash (2%)
Other concerns related to adverse effects are:
- Recurrence of Clostridium difficile associated diarrhea[19]
- Osteoporosis-related fractures[20][21]
- Hypomagnesemia[22]
Concern has been expressed regarding vitamin B12[23] and iron malabsorption,[24] but effects seem to be insignificant, especially when supplement therapy is provided.[25]
Since their introduction, proton-pump inhibitors (PPIs, especially omeprazole) have also been associated with several cases of acute interstitial nephritis,[26] an inflammation of the kidneys that often occurs as an adverse drug reaction.
Long-term use of PPIs is strongly associated with the development of benign polyps from fundic glands (which is distinct from fundic gland polyposis); these polyps do not cause cancer and resolve when PPIs are discontinued. No association is seen between PPI use and cancer, but use of PPIs may mask gastric cancers or other serious gastric problems and physicians should be aware of this effect.[27]
There is a tentative association between long term use and dementia which requires further study to confirm.[28]
Pregnancy and breast-feeding
The safety of using omeprazole has not been established in pregnant or breast-feeding women.[9] Epidemiological data do not show an increased risk of major birth defects after maternal use of omeprazole during pregnancy.[29]
No clinical trials have deeply evaluated the potential consequences of the use of omeprazole in breastfeeding. However, the pharmacokinetics of the omeprazole molecule strongly suggest the safety of omeprazole use during breastfeeding:
- Omeprazole has a high plasma protein binding rate (95%),[30] indicating that little amount of drug is transferred to the milk duct during breast milk formation.
- Omeprazole needs to be administrated in an enteric-coated formulation due to its rapid degradation in the acidic conditions of the stomach. This suggests that most of the free molecules ingested by the infant are likely degraded before being absorbed.
Omeprazole at normal doses is likely safe during breastfeeding.[31]
Interactions
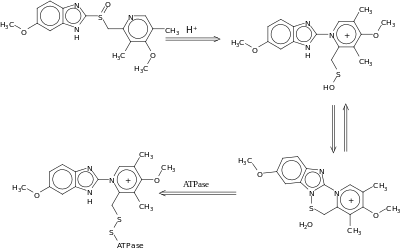
Important drug interactions are rare.[32][33] However, the most significant major drug interaction concern is the decreased activation of clopidogrel when taken together with omeprazole.[34] Although still controversial,[35] this may increase the risk of stroke or heart attack in people taking clopidogrel to prevent these events.
This interaction is possible because omeprazole is an inhibitor of the enzymes CYP2C19 and CYP3A4.[36] Clopidogrel is an inactive prodrug that partially depends on CYP2C19 for conversion to its active form. Inhibition of CYP2C19 may block the activation of clopidogrel, which could reduce its effects.[37][38]
Almost all benzodiazepines are metabolised by the CYP3A4 and CYP2D6 pathways, and inhibition of these enzymes results in a higher AUC (i.e., the total effect over time of a given dose). Other examples of drugs dependent on CYP3A4 for their metabolism are escitalopram,[39] warfarin,[40] oxycodone, tramadol, and oxymorphone. The concentrations of these drugs may increase if they are used concomitantly with omeprazole.[41]
Omeprazole is also a competitive inhibitor of p-glycoprotein, as are other PPIs.[42]
Drugs that depend on an acidic stomach environment (such as ketoconazole or atazanavir) may be poorly absorbed, whereas acid-labile antibiotics (such as erythromycin which is a very strong CYP3A4 inhibitor) may be absorbed to a greater extent than normal due to the more alkaline environment of the stomach.[41]
St. John's wort (Hypericum perforatum) and Gingko biloba significantly reduce plasma concentrations of omeprazole through induction of CYP3A4 and CYP2C19.[43]
Proton-pump inhibitors like omeprazole have been found to increase the plasma concentrations of methotrexate.[44]
Pharmacology
Omeprazole irreversibly blocks the enzyme system on parietal cells that is needed for secretion of gastric acid. It is a specific H+/K+ATPase inhibitor. This is the enzyme needed for the final step in secretion of gastric acid.[45]
Mechanism of action
Omeprazole is a selective and irreversible proton pump inhibitor. It suppresses stomach acid secretion by specific inhibition of the H+/K+-ATPase system found at the secretory surface of gastric parietal cells. Because this enzyme system is regarded as the acid (proton, or H+) pump within the gastric mucosa, omeprazole inhibits the final step of acid production.[45]
Omeprazole also inhibits both basal and stimulated acid secretion irrespective of the stimulus[46] as it blocks the last step in acid secretion.[46] The drug binds non-competitively so it has a dose dependent effect.[47]
The inhibitory effect of omeprazole occurs within 1 hour after oral administration. The maximum effect occurs within 2 hours. The duration of inhibition is up to 72 hours. When omeprazole is stopped, baseline stomach acid secretory activity returns after 3 to 5 days. The inhibitory effect of omeprazole on acid secretion will plateau after 4 days of repeated daily dosing.[48]
Pharmacokinetics
The absorption of omeprazole takes place in the small intestine and is usually completed within 3 to 6 hours. The systemic bioavailability of omeprazole after repeated doses is about 60%.[49] Omeprazole has a volume of distribution of 0.4 L/kg. It has high plasma protein binding of 95%.[47]
Omeprazole, as well as other PPIs, are only effective on active H+/K+-ATPase pumps. These pumps are stimulated in the presence of food to aid in digestion. For this reason, patients should be advised to take omeprazole with a glass of water on an empty stomach.[50] Additionally, most sources recommend that after taking omeprazole, at least 30 minutes should be allowed to elapse before eating[51][52] (at least 60 minutes for immediate-release omeprazole plus sodium bicarbonate products, such as Zegerid),[53] though some sources say that with delayed-release forms of omeprazole, waiting before eating after taking the medication is not necessary.[54]
Omeprazole is completely metabolized by the cytochrome P450 system, mainly in the liver, by CYP2C19 and CYP3A4 isoenzymes.[9] Identified metabolites are the sulfone, the sulfide, and hydroxy-omeprazole, which exert no significant effect on acid secretion. About 77% of an orally given dose is excreted as metabolites in the urine, and the remainder is found in the feces, primarily originating from bile secretion.[46] Omeprazole has a half life of 0.5 to 1 hour.[46] The pharmacological effects of omeprazole last longer as it is covalently bonded to proton pump on parietal cells to induce effects.
Chemistry
Omeprazole contains a tricoordinated sulfinyl sulfur in a pyramidal structure and therefore can exist as either the (S)- or (R)-enantiomers. Omeprazole is a racemate, an equal mixture of the two. In the acidic conditions of the canaliculi of parietal cells, both enantiomers are converted to achiral products (sulfenic acid and sulfenamide configurations) which react with a cysteine group in H+/K+ ATPase, thereby inhibiting the ability of the parietal cells to produce gastric acid.
AstraZeneca also developed esomeprazole (Nexium) which is a eutomer, purely the (S)-enantiomer, rather than a racemate like omeprazole.
Omeprazole undergoes a chiral shift in vivo which converts the inactive (R)-enantiomer to the active (S)-enantiomer, doubling the concentration of the active form.[55] This chiral shift is accomplished by the CYP2C19 isozyme of cytochrome P450, which is not found equally in all human populations. Those who do not metabolize the drug effectively are called "poor metabolizers". The proportion of the poor metabolizer phenotype varies widely between populations, from 2.0–2.5% in African Americans and white Americans to >20% in Asians. Several pharmacogenomics studies have suggested that PPI treatment should be tailored according to CYP2C19 metabolism status.[56]
Measurement in body fluids
Omeprazole may be quantified in plasma or serum to monitor therapy or to confirm a diagnosis of poisoning in hospitalized patients. Plasma omeprazole concentrations are usually in a range of 0.2–1.2 mg/l in persons receiving the drug therapeutically by the oral route and 1–6 mg/l in victims of acute overdose. Enantiomeric chromatographic methods are available to distinguish esomeprazole from racemic omeprazole.[57]
History
Omeprazole was first made in 1979 by Swedish AB Hässle, part of Astra AB. It was the first of the proton pump inhibitors (PPI).[58][59] Astra AB, now AstraZeneca, launched it as an ulcer medicine under the name Losec in Sweden. It was first sold in the United States in 1989 under the brand name Losec. In 1990, at the request of the U.S. Food and Drug Administration, the brand name Losec was changed to Prilosec to avoid confusion with the diuretic Lasix (furosemide).[60] The new name led to confusion between omeprazole (Prilosec) and fluoxetine (Prozac), an antidepressant.[60]
When Prilosec's U.S. patent expired in April 2001, AstraZeneca introduced esomeprazole (Nexium) as a patented replacement drug.[61] Many companies introduced generics as AstraZeneca's patents expired worldwide, which are available under many brand names.
Society and culture
Dosage forms
It can be taken by mouth, as a capsule, tablet, or suspension, or by injection into a vein.[1][8]
Omeprazole is available in strengths of 10, 20, 40, and in some markets 80 mg; and as a powder (omeprazole sodium) for intravenous injection. Most oral omeprazole preparations are enteric-coated, due to the rapid degradation of the drug in the acidic conditions of the stomach. This is most commonly achieved by formulating enteric-coated granules within capsules, enteric-coated tablets, and the multiple-unit pellet system (MUPS).[62] An immediate release formulation was approved by the FDA in the United States,[63] which does not require enteric coating.
It is also available for use in injectable form (IV) in Europe, but not in the U.S. The injection pack is a combination pack consisting of a vial and a separate ampule of reconstituting solution. Each 10 ml clear glass vial contains a white to off-white lyophilised powder consisting of omeprazole sodium 42.6 mg, equivalent to 40 mg of omeprazole.
Omeprazole is also available as an oral suspension of enteric-coated beads in the UK as an unlicensed product. Oral suspensions are predominantly used for children, but can also be used by those with difficulty swallowing or those using a feeding tube.
References
- "Omeprazole". The American Society of Health-System Pharmacists. Retrieved 21 October 2018.
- "Omeprazole international". Drugs.com. 3 February 2020. Retrieved 27 February 2020.
- "Omeprazole Use During Pregnancy". Drugs.com. 11 April 2019. Retrieved 15 February 2020.
- "Prilosec- omeprazole magnesium capsule, delayed release Prilosec- omeprazole magnesium granule, delayed release". DailyMed. 22 December 2016. Retrieved 15 February 2020.
- Vaz-Da-Silva, Manuel; Loureiro, Ana I.; Nunes, Teresa; Maia, Joana; Tavares, Susana; Falcão, Amilcar; Silveira, Pedro; Almeida, Luis; Soares-Da-Silva, Patricio (2005). "Bioavailability and Bioequivalence of Two Enteric-Coated Formulations of Omeprazole in Fasting and Fed Conditions". Clinical Drug Investigation. 25 (6): 391–399. doi:10.2165/00044011-200525060-00004. PMID 17532679. S2CID 22082780. Archived from the original on 13 March 2013. Retrieved 21 October 2018.
- "Omeprazole MSDS". Retrieved 21 October 2018.
- "[99] Comparative effectiveness of proton pump inhibitors | Therapeutics Initiative". 28 June 2016. Retrieved 14 July 2016.
- "Omeprazole 40 mg Powder for Solution for Infusion". EMC. 10 February 2016. Archived from the original on 7 April 2016. Retrieved 21 October 2018.
- Vallerand, A.H.; Sanoski, C.A.; Deglin, J.H. (2015). Davis's Drug Guide for Nurses (14th ed.). F.A. Davis Company. pp. 924–925. ISBN 978-0-8036-4085-6. OCLC 881473728.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 445. ISBN 9783527607495.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- "The Top 300 of 2020". ClinCalc. 23 December 2019. Retrieved 27 February 2020.
- "Omeprazole - Drug Usage Statistics, ClinCalc DrugStats Database". ClinCalc. 23 December 2019. Retrieved 27 February 2020.
- Research, Center for Drug Evaluation and (3 November 2018). "Questions and Answers on Prilosec OTC (omeprazole)". FDA. Retrieved 2 March 2020.
- Cheng, Edaire (21 July 2013). "Proton Pump Inhibitors for Eosinophilic Esophagitis". Current Opinion in Gastroenterology. 29 (4): 416–420. doi:10.1097/MOG.0b013e32835fb50e. ISSN 0267-1379. PMC 4118554. PMID 23449027.
- Fuccio, Lorenzo; Minardi, Maria Eugenia; Zagari, Rocco Maurizo; Grilli, Diego; Magrini, Nicola; Bazzoli, Franco (2007). "Meta-analysis: Duration of First-Line Proton-Pump Inhibitor–Based Triple Therapy for Helicobacter pylori Eradication". Annals of Internal Medicine. 147 (8): 553–62. doi:10.7326/0003-4819-147-8-200710160-00008. PMID 17938394. S2CID 11644009.
- Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ (21 June 2007). "Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report". Gut. 56 (6): 772–81. doi:10.1136/gut.2006.101634. PMC 1954853. PMID 17170018.
- McTavish D, Buckley MM, Heel RC (1991). "Omeprazole. An updated review of its pharmacology and therapeutic use in acid-related disorders". Drugs. 42 (1): 138–70. doi:10.2165/00003495-199142010-00008. PMID 1718683.
- Abou Chakra, CN; et al. (21 June 2014). "Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review". PLOS ONE. 9 (6): e98400. Bibcode:2014PLoSO...998400A. doi:10.1371/journal.pone.0098400. PMC 4045753. PMID 24897375.
- Yang, Yu-Xiao; et al. (2006). "Long-term proton pump inhibitor therapy and risk of hip fracture". JAMA. 296 (24): 2947–2953. doi:10.1001/jama.296.24.2947. PMID 17190895.
- Yu, Elaine W.; et al. (2011). "Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies". The American Journal of Medicine. 124 (6): 519–526. doi:10.1016/j.amjmed.2011.01.007. PMC 3101476. PMID 21605729.
- Hess, M. W.; et al. (2012). "Systematic review: hypomagnesaemia induced by proton pump inhibition". Alimentary Pharmacology & Therapeutics. 36 (5): 405–413. doi:10.1111/j.1365-2036.2012.05201.x. PMID 22762246.
- Neal, Keith; Logan, Richard (2001). "Potential gastrointestinal effects of long‐term acid suppression with proton pump inhibitors". Alimentary Pharmacology & Therapeutics. 15 (7): 1085–6. doi:10.1046/j.1365-2036.2001.0994a.x. PMID 11421886.
- Sarzynski, Erin; et al. (2011). "Association between proton pump inhibitor use and anemia: a retrospective cohort study". Digestive Diseases and Sciences. 56 (8): 2349–2353. doi:10.1007/s10620-011-1589-y. PMID 21318590. S2CID 33574008.
- McColl, Kenneth EL (2009). "Effect of proton pump inhibitors on vitamins and iron". The American Journal of Gastroenterology. 104: S5–S9. doi:10.1038/ajg.2009.45. PMID 19262546. S2CID 31455416.
- Härmark, Linda; et al. (2007). "Proton pump inhibitor‐induced acute interstitial nephritis". British Journal of Clinical Pharmacology. 64 (6): 819–823. doi:10.1111/j.1365-2125.2007.02927.x. PMC 2198775. PMID 17635502.
- Corleto, V.D. (21 February 2014). "Proton pump inhibitor therapy and potential long-term harm". Curr Opin Endocrinol Diabetes Obes. 21 (1): 3–8. doi:10.1097/med.0000000000000031. PMID 24310148.
- Eusebi, LH; Rabitti, S; Artesiani, ML; Gelli, D; Montagnani, M; Zagari, RM; Bazzoli, F (21 July 2017). "Proton pump inhibitors: Risks of long-term use". Journal of Gastroenterology and Hepatology. 32 (7): 1295–1302. doi:10.1111/jgh.13737. PMID 28092694.
- Pasternak, Björn; Hviid, Anders (2010). "Use of Proton-Pump Inhibitors in Early Pregnancy and the Risk of Birth Defects". New England Journal of Medicine. 363 (22): 2114–23. doi:10.1056/NEJMoa1002689. PMID 21105793. S2CID 10954538.
- "Omeprazole drug summary". PDR.net. Archived from the original on 3 March 2014. Retrieved 21 October 2018.
- "LACTMED: OMEPRAZOLE". 10 March 2015. Archived from the original on 8 September 2017. Retrieved 21 October 2018.
- Fitzakerley, Janet. "2014 Treatments for Acid-Peptic Diseases". University of Minnesota Medical School Duluth. Archived from the original on 19 April 2014. Retrieved 21 October 2018.
- "Proton Pump Inhibitor: Use in Adults" (PDF). CMS Medicaid Integrity Program. Archived from the original (PDF) on 12 December 2013. Retrieved 21 October 2018.
- Douglas, I. J.; Evans, S. J.; Hingorani, A. D.; Grosso, A. M.; Timmis, A; Hemingway, H; Smeeth, L (2012). "Clopidogrel and interaction with proton pump inhibitors: comparison between cohort and within person study designs". BMJ. 345: e4388. doi:10.1136/bmj.e4388. PMC 3392956. PMID 22782731.
- Focks, J. J.; Brouwer, M. A.; Van Oijen, M. G. H.; Lanas, A.; Bhatt, D. L.; Verheugt, F. W. A. (2012). "Concomitant use of clopidogrel and proton pump inhibitors: Impact on platelet function and clinical outcome- a systematic review". Heart. 99 (8): 520–7. doi:10.1136/heartjnl-2012-302371. PMID 22851683. S2CID 23689175.
- Shirasaka, Y; Sager, J. E.; Lutz, J. D.; Davis, C; Isoherranen, N (July 2013). "Inhibition of CYP2C19 and CYP3A4 by Omeprazole Metabolites and Their Contribution to Drug-Drug Interactions". Drug Metab. Dispos. 41 (7): 1414–24. doi:10.1124/dmd.113.051722. PMC 3684819. PMID 23620487.
- Lau WC, Gurbel PA (March 2009). "The drug-drug interaction between proton pump inhibitors and clopidogrel". CMAJ. 180 (7): 699–700. doi:10.1503/cmaj.090251. PMC 2659824. PMID 19332744.
- Norgard NB, Mathews KD, Wall GC (July 2009). "Drug-drug interaction between clopidogrel and the proton pump inhibitors". Ann Pharmacother. 43 (7): 1266–1274. doi:10.1345/aph.1M051. PMID 19470853. S2CID 13227312.
- Selective Serotonin Reuptake Inhibitors and CYP2D6 at eMedicine
- Daly AK, King BP (May 2003). "Pharmacogenetics of oral anticoagulants". Pharmacogenetics. 13 (5): 247–52. doi:10.1097/00008571-200305000-00002. PMID 12724615.
- Stedman CA, Barclay ML (August 2000). "Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors". Aliment Pharmacol Ther. 14 (8): 963–978. doi:10.1046/j.1365-2036.2000.00788.x. PMID 10930890.
- Pauli-Magnus C, Rekersbrink S, Klotz U, Fromm MF (December 2001). "Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein". Naunyn Schmiedebergs Arch Pharmacol. 364 (6): 551–557. doi:10.1007/s00210-001-0489-7. PMID 11770010. S2CID 19990184.
- Izzo, AA; Ernst, E (2009). "Interactions between herbal medicines and prescribed drugs: an updated systematic review". Drugs. 69 (13): 1777–1798. doi:10.2165/11317010-000000000-00000. PMID 19719333. S2CID 25720882.
- Brayfield, A, ed. (6 January 2014). "Methotrexate". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 12 April 2014.
- Howden, CW (January 1991). "Clinical pharmacology of omeprazole". Clinical Pharmacokinetics. 20 (1): 38–49. doi:10.2165/00003088-199120010-00003. PMID 2029801. S2CID 25855436.
- "Omeprazole". www.drugbank.ca. Retrieved 29 January 2019.
- Clissold, Stephen P.; Campoli-Richards, Deborah M. (July 1986). "Omeprazole". Drugs. 32 (1): 15–47. doi:10.2165/00003495-198632010-00002. PMID 3527658.
- Omeprazole [package insert]. Archived 19 April 2014 at the Wayback Machine India: Dr. Reddy's Laboratories Limited. Revised: 0613
- Cederberg, C.; Andersson, T.; Skånberg, I. (1 January 1989). "Omeprazole: Pharmacokinetics and Metabolism in Man". Scandinavian Journal of Gastroenterology. 24 (sup166): 33–40. doi:10.3109/00365528909091241. ISSN 0036-5521. PMID 2690330.
- Katz, PO; Gerson, LB; Vela, MF (2013). "Guidelines for the diagnosis and management of gastroesophageal reflux disease". Am J Gastroenterol. 108 (3): 308–28. doi:10.1038/ajg.2012.444. PMID 23419381. S2CID 8198975.
- "Omeprazole, in The Free Medical Dictionary". Retrieved 11 November 2010.
- "Omeprazole". Drugs.com. Archived from the original on 19 February 2011. Retrieved 11 November 2010.
- "Zegird, How to take". rxlist.com. Archived from the original on 26 October 2010. Retrieved 11 November 2010.
- essential drug information. MIMS USA. Retrieved 20 December 2009.
- Nexium Prescribing Information Archived 8 October 2009 at the Wayback Machine. AstraZeneca Pharmaceuticals.
- Furuta T, Shirai N, Sugimoto M, Nakamura A, Hishida A, Ishizaki T (June 2005). "Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies". Drug Metab Pharmacokinet. 20 (3): 153–67. doi:10.2133/dmpk.20.153. PMID 15988117.
- Baselt RC, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1146–7. ISBN 978-0-9626523-7-0.
- "Trends in Drug Patenting - Case Studies: THE CASES: 5. OMEPRAZOLE". apps.who.int. Retrieved 22 August 2019.
- Fellenius, Erik; Berglindh, Thomas; Sachs, George; Olbe, Lars; Elander, Berit; Sjöstrand, Sven-Erik; Wallmark, Björn (1981). "Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+) ATPase". Nature. 290 (5802): 159–61. Bibcode:1981Natur.290..159F. doi:10.1038/290159a0. PMID 6259537. S2CID 4368190.
- Farley, D (July–August 1995). "Making it easier to read prescriptions". FDA Consum. 29 (6): 25–7. PMID 10143448. Archived from the original on 15 March 2012. Retrieved 26 January 2011.
- Gardiner Harris (6 June 2002). "Prilosec's Maker Switches Users To Nexium, Thwarting Generics". The Wall Street Journal. Archived from the original on 6 August 2017.
- Jerome Aubert*, Chris JJ Mulder†, Karsten Schrör**, Stephan R Vavricka††. "Omeprazole MUPS®: An Advanced Formulation offering Flexibility and Predictability for Self Medication." Archived 11 June 2016 at the Wayback Machine SelfCare Journal 2 (2011): 0-0.
- Santarus. Santarus Receives FDA Approval for Immediate-Release Omeprazole Tablet with Dual Buffers. Archived 18 April 2014 at Archive.today N.p., 4 December 2009. Web. 18 April 2014.
Further reading
- Dean L (2012). "Omeprazole Therapy and CYP2C19 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520353. Bookshelf ID: NBK100895.
External links
| Wikimedia Commons has media related to Omeprazole. |
- "Omeprazole". Drug Information Portal. U.S. National Library of Medicine.