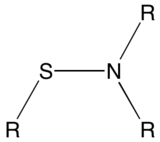
Sulfenamide
Sulfenamides (also spelled sulphenamides) are a class of organosulfur compounds characterized by the general formula RSNR'2, where R and R' are H, alkyl, or aryl.[1] Sulfenamides have been used extensively in the vulcanization of rubber using sulfur. They are related to the oxidized compounds sulfinamides (RS(O)NR'2) and sulfonamides (RS(O)2NR'2).
Preparation
Sulfenamides are usually prepared by the reaction of sulfenyl chlorides and amines:[2]
- RSCl + R'2NH → RSNR'2 + HCl
The S-N bond formation generally obeys standard bimolecular nucleophilic substitution rules, with the basic nitrogen centre being the nucleophile. Primary sulfenamide formation as shown above occurs with the reaction of the sulfenyl halide with ammonia. Additionally primary as well as secondary and tertiary amines form sulfenamides through reaction with, thiols, disulfides, and sulfenyl thiocyanates.[3] In one illustrative synthesis, triphenylmethanesulphenyl chloride and butylamine react in benzene at 25 C:
- Ph3CSCl + 2BuNH2 → Ph3CSN(H)Bu + BuNH3Cl
Many other routes to sulfenamides are known, starting from thiols and disulfides.[4]
- RSSR + 2R'2NH + Ag+ → RSNR'2 + AgSR + R'2NH2+
Structure
Sulfenamides have been characterized by X-ray crystallography. The S-N bond in sulfenamides is a chiral axis that leads to formation of diastereomeric compounds. The existence of these distinct stereoisomers is due to the formation of a partial double bond between either sulfur or nitrogen’s lone pair and the other atom's antibonding orbitals.[1] Additionally bulky substituent groups and lone pair repulsion can contribute resistance to interconversion. The resulting torsional barriers can be quite large and vary from 12-20 kcal/mol.[2] The interactions are thought to be dependent on the torsional preferences (also known as the gauche effect).[1] The nitrogen atom is usually pyramidal, but cyclic and strongly steric hindered acyclic sulfenamides can display a planar arrangement of bonds around the nitrogen atom.
Reactions
The S-N bond n sulfenamide are labile in a variety of ways.[2] The sulfur atom tends to be the more electrophilic center of the S-N bond. Nucleophillic attack on sulfur can occur by amines, by thiols, and by alkyl-magnesium halides which leads to either new sulfenamide compounds or back to starting compounds such as sulfides and disulfides respectively.[1] Both the nitrogen and sulfur atoms comprising the S-N bond in sulfenamides have lone pairs of electrons in their outer shells, one and two for nitrogen and sulfur respectively. These lone pairs allow for the possibility of forming either higher order bonds(double, triple) or adding new subsituent groups to the compound For instance the nitrogen in the S-N bond of 2-hydroxysulfenanilides can oxidized to an imine species with sodium dichromate.[2]
Sulfenamides react with amino-azaheterocycles to form heterocyclic systems (often used as amino protecting groups in various other synthesis reactions). Chlorocarbonylsulfenyl chloride (ClCOSCl) also readily forms S-N bonds with 2-amino-azaheterocycles, but always of a cyclical nature.
A novel variant of the Appel reaction has been noted for sulfenamides. Reaction of o-nitrobenzenesulfenamide with PPh3 and CCl4 leads the formation of o-nitro-N-(triphenylphosphorany1idene)-benzenesulfenamide. In this variant reaction the triphenyl phosphine forms a double bonded linkage with nitrogen in the sulfenamide instead of oxygen as is customary in the Appel reaction. Additionally in the traditional Apple reaction the R-OH bond is cleaved leaving oxygen attached to triphenylphosphine. In this variant the S-N bond is not cleaved.
Applications
Sulfenamides, e.g. cyclohexylthiophthalimide, are used extensively in the vulcanization of rubber. The sulfenamides are used to accelerate the process via the transient formation of labile S-N bonds. The substituents on the sulfenamides determine the point at which they will become active. Temperature dependent activation of sulfenamide accelerants is useful in the vulcanization process because the temperature at which the rubber polymerizes determines the length of the sulfur chains, and properties such as the elasticity of the final product.
References
- Capozzi, G., Modena, G., Pasquato, L. in "Chemistry of Sulphenyl Halides and Sulfenamides" The Chemistry of Sulphenic Acids and their derivatives. Ed. Saul Patai. John Wiley & Sons Ltd. Chapter 10. 403-516, 1990. doi:10.1002/9780470772287
- Craine, Leslie; Raban, Morton (1989). "The Chemistry of Sulfenamides". Chemical Reviews. 89: 669. doi:10.1021/cr00094a001.
- Drabowicz, J., Kielbasinski, P., Mikoiajczyk, M. (1990). "Synthesis of Sulphenyl Halides and Sulphenamides". The Chemistry of Sulphenic Acids and their derivatives. Ed. Saul Patai. John Wiley & Sons Ltd.. Chapter 6. 221-292. doi:10.1002/9780470772287
- I V Koval' "Synthesis and Application of Sulfenamides" Russian Chemical Reviews, 1996, Volume 65, doi:10.1070/RC1996v065n05ABEH000218