Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.[1]

Synthesis and reactions
By oxidation of thioethers and sulfoxides
Sulfones are typically prepared by organic oxidation of thioethers, often referred to as sulfides. Sulfoxides are intermediates in this route.[2] For example, dimethyl sulfide oxidizes to dimethyl sulfoxide and then to dimethyl sulfone.[1]
From SO2
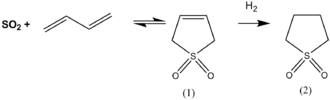 Synthesis of sulfolane by hydrogenation of sulfolene.
Synthesis of sulfolane by hydrogenation of sulfolene.
Sulfur dioxide is a convenient and widely used source of the sulfonyl functional group. Specifically, Sulfur dioxide participates in cycloaddition reactions with dienes.[3] The industrially useful solvent sulfolane is prepared by addition of sulfur dioxide to buta-1,3-diene followed by hydrogenation of the resulting sulfolene.[4]
From sulfonyl and sulfuryl halides
Sulfones are prepared under conditions used for Friedel–Crafts reactions using sources of RSO2+ derived from sulfonyl halides and sulfonic acid anhydrides. Lewis acid catalysts such as AlCl3 and FeCl3 are required.[5][6][7]
Sulfones have been prepared by nucleophilic displacement of halides by sulfinates:[8]
- ArSO2Na + Ar'Cl → Ar(Ar')SO2 + NaCl
Reactions
Sulfone is a relatively inert functional group, being weakly basic (compared to sulfoxides). They are non-oxidizing. In the Ramberg–Bäcklund reaction and the Julia olefination, sulfones are converted to alkenes by the elimination of sulfur dioxide.[9]
Applications
Sulfolane is used to extract valuable aromatic compounds from petroleum.[4]
Polymers
Some polymers containing sulfone groups are useful engineering plastics. They exhibit high strength and resistance to oxidation, corrosion, high temperatures, and creep under stress. For example, some are valuable as replacements for copper in domestic hot water plumbing.[10] Precursors to such polymers are the sulfones bisphenol S and 4,4′-dichlorodiphenyl sulfone.
Pharmacology
Examples of sulfones in pharmacology include dapsone, a drug formerly used as an antibiotic to treat leprosy, dermatitis herpetiformis, tuberculosis, or pneumocystis pneumonia (PCP). Several of its derivatives, such as promin, have similarly been studied or actually been applied in medicine, but in general sulfones are of far less prominence in pharmacology than for example the sulfonamides.[11][12]
See also
- Organosulfur chemistry
- Sulfonanilide
- Sulfoxide
- Sulfonic acid (–OH substituent)
References
| Wikimedia Commons has media related to Sulfones. |
- Hornback, Joseph (2006). Organic Chemistry. Australia: Thomson Brooks/Cole. ISBN 978-0-534-38951-2.
- Leo A. Paquette, Richard V. C. Carr (1986). "Phenyl Vinyl Sulfone and Sulfoxide". Org. Synth. 64: 157. doi:10.15227/orgsyn.064.0157.
- Robert L. Frank and Raymond P. Seven (1949). "Isoprene Cyclic Sulfone". Org. Synth. 29: 59. doi:10.15227/orgsyn.029.0059.
- Folkins, Hillis O. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
- Truce, W. E.; Vriesen; C. W. (1953). "Friedel—Crafts Reactions of Methanesulfonyl Chloride with Benzene and Certain Substituted Benzenes". J. Am. Chem. Soc. 75 (20): 5032–5036. doi:10.1021/ja01116a043.
- Répichet, S.; Le Roux, C.; Hernandez, P.; Dubac, J.; Desmurs, J. R. (1999). "Bismuth(III) Trifluoromethanesulfonate: An Efficient Catalyst for the Sulfonylation of Arenes". The Journal of Organic Chemistry. 64 (17): 6479–6482. doi:10.1021/jo9902603.
- Truce, W. E.; Milionis, J. P. (1952). "Friedel-Crafts Cyclization of ω-Phenylalkanesulfonyl Chlorides". J. Am. Chem. Soc. 74 (4): 974–977. doi:10.1021/ja01124a031.
- C. W. Ferry, J. S. Buck, R. Baltzly (1942). "4,4'-Diaminodiphenylsulfone". Org. Synth. 22: 31. doi:10.15227/orgsyn.022.0031.CS1 maint: multiple names: authors list (link)
- Carey, Francis A.; Sundberg, Richard J. (2007). Advanced Organic Chemistry. Berlin: Springer. ISBN 978-0-387-68354-6.
- Fink, Johannes (2008). High Performance Polymers. Norwich: William Andrew. ISBN 978-0-8155-1580-7.
- Craig, Charles R.; Stitzel, Robert E. (2004). Modern Pharmacology with Clinical Applications. Hagerstwon: Lippincott Williams & Wilkins. ISBN 978-0-7817-3762-3.
- Drill, Victor Alexander; Di Palma, Joseph R. (1971). Drill's Pharmacology in Medicine. New York: McGraw-Hill. ISBN 978-0-07-017006-3.