Triazavirin
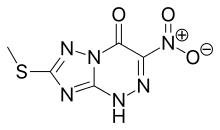
Triazavirin (TZV, Riamilovir) is a broad-spectrum antiviral drug developed in Russia through a joint effort of Ural Federal University, Russian Academy of Sciences, Ural Center for Biopharma Technologies and Medsintez Pharmaceutical. It has a novel triazolotriazine core, which represents a new structural class of non-nucleoside antiviral drugs.[1]
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.074 |
| Chemical and physical data | |
| Formula | C5H4N6O3S |
| Molar mass | 228.189 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Uses
It was originally developed as a potential treatment for pandemic influenza strains such as H5N1, and most of the testing that has been done has focused on its anti-influenza activity.[2][3][4] However triazavirin has also been found to have antiviral activity against a number of other viruses including Tick-borne encephalitis virus,[5] and Forest-Spring Encephalitis virus,[6] and is also being investigated for potential application against Lassa fever and Ebola virus disease.[7][8][9][10][11] In February 2020, testing of triazavirin was started against SARS-CoV-2.[12][13][14]
See also
References
- Rusinov VL, Sapozhnikova IM, Ulomskii EN, Medvedeva NR, Egorov VV, Kiselev OI, et al. (2015). "Nucleophilic substitution of nitro group in nitrotriazolotriazines as a model of potential interaction with cysteine-containing proteins". Chemistry of Heterocyclic Compounds. 51 (3): 275–280. doi:10.1007/s10593-015-1695-4.
- Loginova SI, Borisevich SV, Maksimov VA, Bondarev VP, Kotovskaia SK, Rusinov VL, et al. (2007). "[Investigation of triazavirin antiviral activity against influenza A virus (H5N1) in cell culture]". Antibiotiki I Khimioterapiia = Antibiotics and Chemoterapy [Sic] (in Russian). 52 (11–12): 18–20. PMID 19275052.
- Karpenko I, Deev S, Kiselev O, Charushin V, Rusinov V, Ulomsky E, et al. (May 2010). "Antiviral properties, metabolism, and pharmacokinetics of a novel azolo-1,2,4-triazine-derived inhibitor of influenza A and B virus replication". Antimicrobial Agents and Chemotherapy. 54 (5): 2017–22. doi:10.1128/AAC.01186-09. PMC 2863629. PMID 20194696.
- Kiselev OI, Deeva EG, Mel'nikova TI, Kozeletskaia KN, Kiselev AS, Rusinov VL, et al. (2012). "[A new antiviral drug Triazavirin: results of phase II clinical trial]". Voprosy Virusologii (in Russian). 57 (6): 9–12. PMID 23477247.
- Loginova SI, Borisevich SV, Rusinov VL, Ulomskiĭ UN, Charushin VN, Chupakhin ON (2014). "[Investigation of Triazavirin antiviral activity against tick-borne encephalitis pathogen in cell culture]". Antibiotiki I Khimioterapiia = Antibiotics and Chemoterapy [Sic] (in Russian). 59 (1–2): 3–5. PMID 25051708.
- Loginova SY, Borisevich SV, Rusinov VL, Ulomsky EN, Charushin VN, Chupakhin ON, Sorokin PV (2015). "[Investigation of Therapeutic Efficacy of Triazavirin Against Experimental Forest-Spring Encephalitis on Albino Mice]". Antibiotiki I Khimioterapiia = Antibiotics and Chemoterapy [Sic] (in Russian). 60 (7–8): 11–3. PMID 26863736.
- "Target: Ebola". Pravda. 2014-12-22. Retrieved 18 January 2015.
- "Yekaterinburg pharmacies to sell domestic antiviral drug". Retrieved 18 January 2015.
- Cox S (2014-10-17). "Ebola crisis: Vaccine 'too late' for outbreak. BBC News, 17 October 2014". BBC News.
- Kukil Bora. Russia Will Begin Testing Triazavirin, Used For Lassa Fever, And Other Drugs On Ebola: Health Ministry. International Business Times, 12 November 2014
- Kezina, Darya (12 November 2014). "New antiviral drug from Urals will help fight Ebola and other viruses". Russia Beyond the Headlines.
- Jamshaid U (4 February 2020). "China Testing Russia's Triazavirin As Coronavirus Treatment". Russian Health Ministry. Urdupoint.
- "China tests Russian anti-viral drug which might treat coronavirus as Moscow warns of possible 'mass outbreak'". Retrieved 11 February 2020.
- "China Tests Russian Antiviral Drug Which Might Treat Coronavirus As Moscow Warns Of Possible 'Mass Outbreak'". 2020-02-05. Retrieved 11 February 2020.