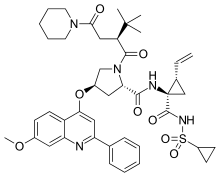
Sovaprevir
Sovaprevir (codenamed ACH-1625) is an experimental drug designed to treat the hepatitis C virus. It is under development by Achillion Pharmaceuticals. It acts as a NS3/4A inhibitor.[1]
 | |
| Clinical data | |
|---|---|
| Other names | ACH-1625 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C43H53N5O8S |
| Molar mass | 799.98 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sovaprevir received Fast Track status from the U.S. Food and Drug Administration (FDA) in 2012.[2]
References
- De Clercq E (June 2014). "Current race in the development of DAAs (direct-acting antivirals) against HCV". Biochemical Pharmacology. 89 (4): 441–52. doi:10.1016/j.bcp.2014.04.005. PMID 24735613.
- "Sovaprevir HCV NS3/4A Protease Inhibitor". Achillion.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.