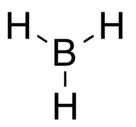
Borane
Trihydridoboron, also known as borane or borine, is an unstable and highly reactive molecule with the chemical formula BH
3. The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule.[1] However, the molecular species BH3 is a very strong Lewis acid. Consequently it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen.[2]
 | |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
borane (substitutive) trihydridoboron (additive) | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| 44 | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| BH3 | |||
| Molar mass | 13.83 g·mol−1 | ||
| Appearance | colourless gas | ||
| Conjugate acid | Boronium | ||
| Thermochemistry | |||
Std molar entropy (S |
187.88 kJ mol−1 K−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
106.69 kJ mol−1 | ||
| Structure | |||
| D3h | |||
| trigonal planar | |||
| 0 D | |||
| Related compounds | |||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Structure and properties
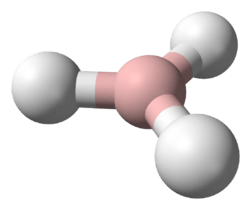
BH3 is trigonal planar molecule with D3h symmetry The experimentally determined B–H bond length is 119 pm.[3]
In the absence of other chemical species, it reacts with itself to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction:[4]
- BX3 +BH4− → HBX3− + (BH3) (X=F, Cl, Br, I)
- 2 BH3 → B2H6
The standard enthalpy of dimerization of BH3 is estimated to be −170 kJ mol−1.[5] The boron atom in BH3 has 6 valence electrons. Consequently it is a strong Lewis acid and reacts with any Lewis base, L to form an adduct.
- BH3 + L → L—BH3
in which the base donates its lone pair, forming a dative covalent bond. Such compounds are thermodynamically stable, but may be easily oxidised in air. Solutions containing borane dimethylsulfide and borane–tetrahydrofuran are commercially available; in tetrahydrofuran a stabilising agent is added to prevent the THF from oxidising the borane.[6] A stability sequence for several common adducts of borane, estimated from spectroscopic and thermochemical data, is as follows:
BH3 has some soft acid characteristics as sulfur donors form more stable complexes than do oxygen donors.[4] Aqueous solutions of BH3 are extremely unstable.[7][8]
- BH
3 + 3H
2O → B(OH)
3 + 3 H
2
Reactions
Molecular BH3 is believed to be a reaction intermediate in the pyrolysis of diborane to produce higher boranes:[4]
- B2H6 ⇌ 2BH3
- BH3 +B2H6 → B3H7 +H2 (rate determining step)
- BH3 + B3H7 ⇌ B4H10
- B2H6 + B3H7 → BH3 + B4H10
- ⇌ B5H11 + H2
Further steps give rise to successively higher boranes, with B10H14 as the most stable end product contaminated with polymeric materials, and a little B20H26.
Borane ammoniate, which is produced by a displacement reaction of other borane adducts, eliminates elemental hydrogen on heating to give borazine (HBNH)3.[9]
Borane adducts are widely used in organic synthesis for hydroboration, where BH3 adds across the C=C bond in alkenes to give trialkylboranes:
- (THF)BH3 + 3 CH2=CHR → B(CH2CH2R)3 + THF
This reaction is regioselective, Other borane derivatives can be used to give even higher regioselectivity.[10] The product trialkylboranes can be converted to useful organic derivatives. With bulky alkenes one can prepare species such as [HBR2]2, which are also useful reagents in more specialised applications. Borane dimethylsulfide which is more stable than borane–tetrahydrofuran may also be used.[11][10]
Hydroboration can be coupled with oxidation to give the hydroboration-oxidation reaction. In this reaction, the boryl group in the generated organoborane is substituted with a hydroxyl group.
Reductive amination is an extension of the hydroboration-oxidation reaction, wherein a carbon–nitrogen double bond is undergoing hydroboration. The carbon–nitrogen double bond is created by the reductive elimination of water from a hemiaminal, formed by the addition of an amine to a carbonyl molecule, hence the adjective 'reductive'.
Borane(5)
Borane(5) is the dihydrogen complex of borane. Its molecular formula is BH5 or possibly BH3(η2-H2).[12] It is only stable at very low temperatures and its existence is confirmed in very low temperature.[13][14] Borane(5) and methanium (CH5+) are isoelectronic.[15] Its conjugate base is the borohydride anion.
References
- Burg, Anton B.; Schlesinger, H. I. (May 1937). "Hydrides of boron. VII. Evidence of the transitory existence of borine (BH
3): Borine carbonyl and borine trimethylammine". Journal of the American Chemical Society. 59 (5): 780–787. doi:10.1021/ja01284a002. - Tague, Thomas J.; Andrews, Lester (1994). "Reactions of Pulsed-Laser Evaporated Boron Atoms with Hydrogen. Infrared Spectra of Boron Hydride Intermediate Species in Solid Argon". Journal of the American Chemical Society. 116 (11): 4970–4976. doi:10.1021/ja00090a048. ISSN 0002-7863.
- Kawaguchi, Kentarou (1992). "Fourier transform infrared spectroscopy of the BH3 ν3 band". The Journal of Chemical Physics. 96 (5): 3411. Bibcode:1992JChPh..96.3411K. doi:10.1063/1.461942. ISSN 0021-9606.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Page, M.; Adams, G.F.; Binkley, J.S.; Melius, C.F. (1987). "Dimerization energy of borane". J. Phys. Chem. 91 (11): 2675–2678. doi:10.1021/j100295a001.
- Hydrocarbon Chemistry, George A. Olah, Arpad Molner, 2d edition, 2003, Wiley-Blackwell ISBN 978-0471417828
- Finn, Patricia; Jolly, William L. (August 1972). "Asymmetric cleavage of diborane by water. The structure of diborane dihydrate". Inorganic Chemistry. 11 (8): 1941–1944. doi:10.1021/ic50114a043.
- D'Ulivo, Alessandro (May 2010). "Mechanism of generation of volatile species by aqueous boranes". Spectrochimica Acta Part B: Atomic Spectroscopy. 65 (5): 360–375. doi:10.1016/j.sab.2010.04.010.
- Housecroft, C. E.; Sharpe, A. G. (2008). "Chapter 13: The Group 13 Elements". Inorganic Chemistry (3rd ed.). Pearson. p. 336. ISBN 978-0-13-175553-6.
- Burkhardt, Elizabeth R.; Matos, Karl (July 2006). "Boron reagents in process chemistry: Excellent tools for selective reductions". Chemical Reviews. 106 (7): 2617–2650. doi:10.1021/cr0406918.
- Kollonitisch, J. (1961). "Reductive Ring Cleavage of Tetrahydrofurans by Diborane". J. Am. Chem. Soc. 83 (6): 1515. doi:10.1021/ja01467a056.
- Szieberth, Dénes; Szpisjak, Tamás; Turczel, Gábor; Könczöl, László (19 August 2014). "The stability of η2-H2 borane complexes – a theoretical investigation". Dalton Transactions. 43 (36): 13571–13577. doi:10.1039/C4DT00019F. PMID 25092548.
- Tague, Thomas J.; Andrews, Lester (1 June 1994). "Reactions of Pulsed-Laser Evaporated Boron Atoms with Hydrogen. Infrared Spectra of Boron Hydride Intermediate Species in Solid Argon". Journal of the American Chemical Society. 116 (11): 4970–4976. doi:10.1021/ja00090a048.
- Schreiner, Peter R.; Schaefer III, Henry F.; Schleyer, Paul von Ragué (1 June 1994). "The structure and stability of BH5. Does correlation make it a stable molecule? Qualitative changes at high levels of theory". The Journal of Chemical Physics. 101 (9): 7625. Bibcode:1994JChPh.101.7625S. doi:10.1063/1.468496.
- A Life of Magic Chemistry: Autobiographical Reflections Including Post-Nobel Prize Years and the Methanol Economy, 159p