FANCD2
Fanconi anemia group D2 protein is a protein that in humans is encoded by the FANCD2 gene.[5][6] The Fanconi anemia complementation group (FANC) currently includes FANCA, FANCB, FANCC, FANCD1 (also called BRCA2), FANCD2 (this gene), FANCE, FANCF, FANCG, FANCI , FANCJ, FANCL, FANCM, FANCN and FANCO.
Function
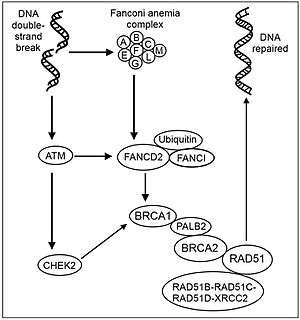
Fanconi anemia is a genetically homozygous recessive disorder characterized by chromosomal instability, hypersensitivity to DNA crosslinking agents, increased chromosomal breakage, and defective DNA repair. The members of the Fanconi anemia complementation group do not share sequence similarity; they are related by their assembly into a common nuclear protein complex. This gene encodes the protein for complementation group D2. This protein is monoubiquitinated in response to DNA damage, resulting in its localization to nuclear foci with other proteins (BRCA1 and BRCA2) involved in homology-directed DNA repair (see Figure: Recombinational repair of DNA double-strand damages). A nuclear complex containing FANCA, FANCA, FANCB,FANCC, FANCE, FANCF, FANCL and FANCG proteins is required for the activation of the FANCD2 protein to the mono-ubiquitinated isoform.[14]
Mono-ubiquination of FANCD2 is essential for repairing DNA interstrand crosslinks, and clamps the protein on DNA together with its partner protein FANCI. The monoubiquitinated FANCD2:FANCI complex coats DNA in a filament-like array, potentially as a way to protect DNA associated with stalled replication.[15]
Mono-ubiquitination is also required for interaction with the nuclease FAN1. FAN1 recruitment and its consequent activity restrain DNA replication fork progression and prevent chromosome abnormalities from occurring when DNA replication forks stall.[16] Alternative splicing results in two transcript variants encoding different isoforms.[17]
Infertility
Humans with a FANCD deficiency display hypogonadism, male infertility, impaired spermatogenesis, and reduced female fertility. Similarly, mice deficient in FANCD2 show hypogonadism, impaired fertility and impaired gametogenesis.[18]
In the non-mutant mouse, FANCD2 is expressed in spermatogonia, pre-leptotene spermatocytes, and in spermatocytes in the leptotene, zygotene and early pachytene stages of meiosis.[19] In synaptonemal complexes of meiotic chromosomes, activated FANCD2 protein co-localizes with BRCA1 (breast cancer susceptibility protein).[14] FANCD2 mutant mice exhibit chromosome mis-pairing during the pachytene stage of meiosis and germ cell loss.[20] Activated FANCD2 protein may normally function prior to the initiation of meiotic recombination, perhaps to prepare chromosomes for synapsis, or to regulate subsequent recombination events.[14]
Clinical significance
Tobacco smoke suppresses the expression of FANCD2, which codes for a DNA damage "caretaker" or repair mechanism.[21]
Cancer
FANCD2 mutant mice have a significantly increased incidence of tumors including ovarian, gastric and hepatic adenomas as well as hepatocellular, lung, ovarian and mammary carcinomas.[18][20] Humans with a FANCD2 deficiency have increased acute myeloid leukemia, and squamous cell carcinomas (head and neck squamous cell carcinomas and anogenital carcinomas).[18]
FANCD2 monoubiquitination is also a potential therapeutic target in the treatment of cancer. [22]
Interactions
FANCD2 has been shown to interact with:
- FANCI[23][24]
- Ataxia telangiectasia mutated,[25][26]
- BARD1,[27]
- BRCA1.[26][27]
- BRCA2,[28][29][30]
- FANCE,[29][31][32]
- HTATIP,[30] and
- MEN1.[33]
References
- GRCh38: Ensembl release 89: ENSG00000144554 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000034023 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Whitney M, Thayer M, Reifsteck C, Olson S, Smith L, Jakobs PM, et al. (November 1995). "Microcell mediated chromosome transfer maps the Fanconi anaemia group D gene to chromosome 3p". Nature Genetics. 11 (3): 341–3. doi:10.1038/ng1195-341. PMID 7581463.
- Timmers C, Taniguchi T, Hejna J, Reifsteck C, Lucas L, Bruun D, et al. (February 2001). "Positional cloning of a novel Fanconi anemia gene, FANCD2". Molecular Cell. 7 (2): 241–8. doi:10.1016/S1097-2765(01)00172-1. PMID 11239453.
- D'Andrea AD (May 2010). "Susceptibility pathways in Fanconi's anemia and breast cancer". The New England Journal of Medicine. 362 (20): 1909–19. doi:10.1056/NEJMra0809889. PMC 3069698. PMID 20484397.
- Sobeck A, Stone S, Landais I, de Graaf B, Hoatlin ME (September 2009). "The Fanconi anemia protein FANCM is controlled by FANCD2 and the ATR/ATM pathways". The Journal of Biological Chemistry. 284 (38): 25560–8. doi:10.1074/jbc.M109.007690. PMC 2757957. PMID 19633289.
- Castillo P, Bogliolo M, Surralles J (May 2011). "Coordinated action of the Fanconi anemia and ataxia telangiectasia pathways in response to oxidative damage". DNA Repair. 10 (5): 518–25. doi:10.1016/j.dnarep.2011.02.007. PMID 21466974.
- Stolz A, Ertych N, Bastians H (February 2011). "Tumor suppressor CHK2: regulator of DNA damage response and mediator of chromosomal stability". Clinical Cancer Research. 17 (3): 401–5. doi:10.1158/1078-0432.CCR-10-1215. PMID 21088254.
- Taniguchi T, Garcia-Higuera I, Andreassen PR, Gregory RC, Grompe M, D'Andrea AD (October 2002). "S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51". Blood. 100 (7): 2414–20. doi:10.1182/blood-2002-01-0278. PMID 12239151.
- Park JY, Zhang F, Andreassen PR (August 2014). "PALB2: the hub of a network of tumor suppressors involved in DNA damage responses". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1846 (1): 263–75. doi:10.1016/j.bbcan.2014.06.003. PMC 4183126. PMID 24998779.
- Chun J, Buechelmaier ES, Powell SN (January 2013). "Rad51 paralog complexes BCDX2 and CX3 act at different stages in the BRCA1-BRCA2-dependent homologous recombination pathway". Molecular and Cellular Biology. 33 (2): 387–95. doi:10.1128/MCB.00465-12. PMC 3554112. PMID 23149936.
- Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, et al. (February 2001). "Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway". Molecular Cell. 7 (2): 249–62. doi:10.1016/s1097-2765(01)00173-3. PMID 11239454.
- Tan W, van Twest S, Leis A, Bythell-Douglas R, Murphy VJ, Sharp M, et al. (March 2020). "Monoubiquitination by the human Fanconi anemia core complex clamps FANCI:FANCD2 on DNA in filamentous arrays". eLife. 9. doi:10.7554/eLife.54128. PMC 7156235. PMID 32167469.
- Lachaud C, Moreno A, Marchesi F, Toth R, Blow JJ, Rouse J (February 2016). "Ubiquitinated Fancd2 recruits Fan1 to stalled replication forks to prevent genome instability". Science. 351 (6275): 846–9. Bibcode:2016Sci...351..846L. doi:10.1126/science.aad5634. PMC 4770513. PMID 26797144.
- "Entrez Gene: FANCD2 Fanconi anemia, complementation group D2".
- Parmar K, D'Andrea A, Niedernhofer LJ (July 2009). "Mouse models of Fanconi anemia". Mutation Research. 668 (1–2): 133–40. doi:10.1016/j.mrfmmm.2009.03.015. PMC 2778466. PMID 19427003.
- Jamsai D, O'Connor AE, O'Donnell L, Lo JC, O'Bryan MK (2015). "Uncoupling of transcription and translation of Fanconi anemia (FANC) complex proteins during spermatogenesis". Spermatogenesis. 5 (1): e979061. doi:10.4161/21565562.2014.979061. PMC 4581071. PMID 26413409.
- Houghtaling S, Timmers C, Noll M, Finegold MJ, Jones SN, Meyn MS, Grompe M (August 2003). "Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice". Genes & Development. 17 (16): 2021–35. doi:10.1101/gad.1103403. PMC 196256. PMID 12893777.
- Hays LE, Zodrow DM, Yates JE, Deffebach ME, Jacoby DB, Olson SB, Pankow JF, Bagby GC (May 2008). "Cigarette smoke induces genetic instability in airway epithelial cells by suppressing FANCD2 expression". British Journal of Cancer. 98 (10): 1653–61. doi:10.1038/sj.bjc.6604362. PMC 2391131. PMID 18475298.
- Sharp MF, Murphy VJ, Twest SV, Tan W, Lui J, Simpson KJ, et al. (May 2020). "Methodology for the identification of small molecule inhibitors of the Fanconi Anaemia ubiquitin E3 ligase complex". Scientific Reports. 10 (1): 7959. doi:10.1038/s41598-020-64868-7. PMC 7224301. PMID 32409752.
- Yuan F, El Hokayem J, Zhou W, Zhang Y (September 2009). "FANCI protein binds to DNA and interacts with FANCD2 to recognize branched structures". The Journal of Biological Chemistry. 284 (36): 24443–52. doi:10.1074/jbc.m109.016006. PMC 2782037. PMID 19561358.
- Joo W, Xu G, Persky NS, Smogorzewska A, Rudge DG, Buzovetsky O, et al. (July 2011). "Structure of the FANCI-FANCD2 complex: insights into the Fanconi anemia DNA repair pathway". Science. 333 (6040): 312–6. Bibcode:2011Sci...333..312J. doi:10.1126/science.1205805. PMC 3310437. PMID 21764741.
- Taniguchi T, Garcia-Higuera I, Xu B, Andreassen PR, Gregory RC, Kim ST, et al. (May 2002). "Convergence of the fanconi anemia and ataxia telangiectasia signaling pathways". Cell. 109 (4): 459–72. doi:10.1016/S0092-8674(02)00747-X. PMID 12086603.
- Reuter TY, Medhurst AL, Waisfisz Q, Zhi Y, Herterich S, Hoehn H, et al. (October 2003). "Yeast two-hybrid screens imply involvement of Fanconi anemia proteins in transcription regulation, cell signaling, oxidative metabolism, and cellular transport". Experimental Cell Research. 289 (2): 211–21. doi:10.1016/S0014-4827(03)00261-1. PMID 14499622.
- Vandenberg CJ, Gergely F, Ong CY, Pace P, Mallery DL, Hiom K, Patel KJ (July 2003). "BRCA1-independent ubiquitination of FANCD2". Molecular Cell. 12 (1): 247–54. doi:10.1016/S1097-2765(03)00281-8. PMID 12887909.
- Wang X, Andreassen PR, D'Andrea AD (July 2004). "Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin". Molecular and Cellular Biology. 24 (13): 5850–62. doi:10.1128/MCB.24.13.5850-5862.2004. PMC 480901. PMID 15199141.
- Hussain S, Wilson JB, Medhurst AL, Hejna J, Witt E, Ananth S, et al. (June 2004). "Direct interaction of FANCD2 with BRCA2 in DNA damage response pathways". Human Molecular Genetics. 13 (12): 1241–8. doi:10.1093/hmg/ddh135. PMID 15115758.
- Hejna J, Holtorf M, Hines J, Mathewson L, Hemphill A, Al-Dhalimy M, et al. (April 2008). "Tip60 is required for DNA interstrand cross-link repair in the Fanconi anemia pathway". The Journal of Biological Chemistry. 283 (15): 9844–51. doi:10.1074/jbc.M709076200. PMC 2398728. PMID 18263878.
- Gordon SM, Buchwald M (July 2003). "Fanconi anemia protein complex: mapping protein interactions in the yeast 2- and 3-hybrid systems". Blood. 102 (1): 136–41. doi:10.1182/blood-2002-11-3517. PMID 12649160.
- Pace P, Johnson M, Tan WM, Mosedale G, Sng C, Hoatlin M, et al. (July 2002). "FANCE: the link between Fanconi anaemia complex assembly and activity". The EMBO Journal. 21 (13): 3414–23. doi:10.1093/emboj/cdf355. PMC 125396. PMID 12093742.
- Jin S, Mao H, Schnepp RW, Sykes SM, Silva AC, D'Andrea AD, Hua X (July 2003). "Menin associates with FANCD2, a protein involved in repair of DNA damage". Cancer Research. 63 (14): 4204–10. PMID 12874027.
Further reading
- Hejna JA, Timmers CD, Reifsteck C, Bruun DA, Lucas LW, Jakobs PM, et al. (May 2000). "Localization of the Fanconi anemia complementation group D gene to a 200-kb region on chromosome 3p25.3". American Journal of Human Genetics. 66 (5): 1540–51. doi:10.1086/302896. PMC 1378015. PMID 10762542.
- Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, et al. (February 2001). "Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway". Molecular Cell. 7 (2): 249–62. doi:10.1016/S1097-2765(01)00173-3. PMID 11239454.
- Futaki M, Liu JM (December 2001). "Chromosomal breakage syndromes and the BRCA1 genome surveillance complex". Trends in Molecular Medicine. 7 (12): 560–5. doi:10.1016/S1471-4914(01)02178-5. PMID 11733219.
- Wilson JB, Johnson MA, Stuckert AP, Trueman KL, May S, Bryant PE, et al. (December 2001). "The Chinese hamster FANCG/XRCC9 mutant NM3 fails to express the monoubiquitinated form of the FANCD2 protein, is hypersensitive to a range of DNA damaging agents and exhibits a normal level of spontaneous sister chromatid exchange". Carcinogenesis. 22 (12): 1939–46. doi:10.1093/carcin/22.12.1939. PMID 11751423.
- Grompe M (June 2002). "FANCD2: a branch-point in DNA damage response?". Nature Medicine. 8 (6): 555–6. doi:10.1038/nm0602-555. PMID 12042798.
- Taniguchi T, Garcia-Higuera I, Xu B, Andreassen PR, Gregory RC, Kim ST, et al. (May 2002). "Convergence of the fanconi anemia and ataxia telangiectasia signaling pathways". Cell. 109 (4): 459–72. doi:10.1016/S0092-8674(02)00747-X. PMID 12086603.
- Pace P, Johnson M, Tan WM, Mosedale G, Sng C, Hoatlin M, et al. (July 2002). "FANCE: the link between Fanconi anaemia complex assembly and activity". The EMBO Journal. 21 (13): 3414–23. doi:10.1093/emboj/cdf355. PMC 125396. PMID 12093742.
- Taniguchi T, Garcia-Higuera I, Andreassen PR, Gregory RC, Grompe M, D'Andrea AD (October 2002). "S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51". Blood. 100 (7): 2414–20. doi:10.1182/blood-2002-01-0278. PMID 12239151.
- Tamary H, Bar-Yam R, Zemach M, Dgany O, Shalmon L, Yaniv I (October 2002). "The molecular biology of Fanconi anemia". The Israel Medical Association Journal. 4 (10): 819–23. PMID 12389351.
- Nakanishi K, Taniguchi T, Ranganathan V, New HV, Moreau LA, Stotsky M, et al. (December 2002). "Interaction of FANCD2 and NBS1 in the DNA damage response". Nature Cell Biology. 4 (12): 913–20. doi:10.1038/ncb879. PMID 12447395.
- Goldberg M, Stucki M, Falck J, D'Amours D, Rahman D, Pappin D, et al. (February 2003). "MDC1 is required for the intra-S-phase DNA damage checkpoint". Nature. 421 (6926): 952–6. Bibcode:2003Natur.421..952G. doi:10.1038/nature01445. PMID 12607003.
- Stewart GS, Wang B, Bignell CR, Taylor AM, Elledge SJ (February 2003). "MDC1 is a mediator of the mammalian DNA damage checkpoint". Nature. 421 (6926): 961–6. Bibcode:2003Natur.421..961S. doi:10.1038/nature01446. PMID 12607005.
- Gordon SM, Buchwald M (July 2003). "Fanconi anemia protein complex: mapping protein interactions in the yeast 2- and 3-hybrid systems". Blood. 102 (1): 136–41. doi:10.1182/blood-2002-11-3517. PMID 12649160.
- Jin S, Mao H, Schnepp RW, Sykes SM, Silva AC, D'Andrea AD, Hua X (July 2003). "Menin associates with FANCD2, a protein involved in repair of DNA damage". Cancer Research. 63 (14): 4204–10. PMID 12874027.
- Vandenberg CJ, Gergely F, Ong CY, Pace P, Mallery DL, Hiom K, Patel KJ (July 2003). "BRCA1-independent ubiquitination of FANCD2". Molecular Cell. 12 (1): 247–54. doi:10.1016/S1097-2765(03)00281-8. PMID 12887909.
- Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, et al. (October 2003). "A novel ubiquitin ligase is deficient in Fanconi anemia". Nature Genetics. 35 (2): 165–70. doi:10.1038/ng1241. PMID 12973351.
- Reuter TY, Medhurst AL, Waisfisz Q, Zhi Y, Herterich S, Hoehn H, et al. (October 2003). "Yeast two-hybrid screens imply involvement of Fanconi anemia proteins in transcription regulation, cell signaling, oxidative metabolism, and cellular transport". Experimental Cell Research. 289 (2): 211–21. doi:10.1016/S0014-4827(03)00261-1. PMID 14499622.
- Wang X, Kennedy RD, Ray K, Stuckert P, Ellenberger T, D'Andrea AD (April 2007). "Chk1-mediated phosphorylation of FANCE is required for the Fanconi anemia/BRCA pathway". Molecular and Cellular Biology. 27 (8): 3098–108. doi:10.1128/MCB.02357-06. PMC 1899922. PMID 17296736.