DDB1
DNA damage-binding protein 1 is a protein that in humans is encoded by the DDB1 gene.[5][6][7]
Gene
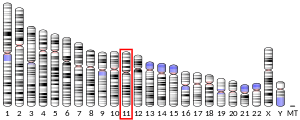
The gene's position is on chromosome 11q12-q13.[8]
Protein
The DDB1 gene encodes the large subunit of DNA damage-binding protein, a heterodimer composed of a large and a small (DDB2) subunit. DDB1 contains 1140 amino acids, amounting to a mass of 127 kDa.[8]
Function
As its name suggests, DDB1 was initially implicated in the process of a specific type of DNA repair known as nucleotide excision repair. Since then, researchers have found that DDB1 primarily functions as a core component of the CUL4A- and CUL4B-based E3 ubiquitin ligase complexes. DDB1 serves as a bridge or adaptor protein which interacts with dozens of proteins known as DDB1 and CUL4-associated factors (DCAFs).[9] These DCAFs are often ubiquitin ligase substrates and regulate numerous essential processes in the cell including DNA repair (DDB2), DNA replication, chromatin remodeling (Cdt2) and more.
Interactions
DDB1 has been shown to interact with Transcription initiation protein SPT3 homolog,[10] GCN5L2,[11] DDB2,[12][13] CUL4A,[13] CUL4B[13] and P21.[14]
References
- GRCh38: Ensembl release 89: ENSG00000167986 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000024740 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Dualan R, Brody T, Keeney S, Nichols AF, Admon A, Linn S (Feb 1996). "Chromosomal localization and cDNA cloning of the genes (DDB1 and DDB2) for the p127 and p48 subunits of a human damage-specific DNA binding protein". Genomics. 29 (1): 62–9. doi:10.1006/geno.1995.1215. PMID 8530102.
- Seki N, Hayashi A, Hattori A, Kozuma S, Sasaki M, Suzuki Y, Sugano S, Muramatsu M, Saito T (Jan 2000). "cDNA cloning, tissue expression, and chromosomal assignment of a mouse gene, encoding a 127 kDa UV-damaged DNA binding protein which is defective in XPE cells". DNA Res. 6 (5): 319–22. doi:10.1093/dnares/6.5.319. PMID 10574459.
- "Entrez Gene: DDB1 damage-specific DNA binding protein 1, 127kDa".
- Iovine, Barbara; Iannella, Maria Luigia; Bevilacqua, Maria Assunta (2011). "Damage-specific DNA binding protein 1 (DDB1): a protein with a wide range of functions". The International Journal of Biochemistry & Cell Biology. Elsevier. 43 (12): 1664–1667. doi:10.1016/j.biocel.2011.09.001. PMID 21959250.
- Lee J (2007). "DCAFs, the Missing Link of the CUL4-DDB1 Ubiquitin Ligase". Molecular Cell. 26 (6): 775–780. doi:10.1016/j.molcel.2007.06.001. PMID 17588513.
- Martinez E, Palhan VB, Tjernberg A, Lymar ES, Gamper AM, Kundu TK, Chait BT, Roeder RG (October 2001). "Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo". Mol. Cell. Biol. 21 (20): 6782–95. doi:10.1128/MCB.21.20.6782-6795.2001. PMC 99856. PMID 11564863.
- Huang J, Chen J (July 2008). "VprBP targets Merlin to the Roc1-Cul4A-DDB1 E3 ligase complex for degradation". Oncogene. 27 (29): 4056–64. doi:10.1038/onc.2008.44. PMID 18332868.
- Bergametti F, Sitterlin D, Transy C (July 2002). "Turnover of hepatitis B virus X protein is regulated by damaged DNA-binding complex". J. Virol. 76 (13): 6495–501. doi:10.1128/JVI.76.13.6495-6501.2002. PMC 136256. PMID 12050362.
- Guerrero-Santoro J, Kapetanaki MG, Hsieh CL, Gorbachinsky I, Levine AS, Rapić-Otrin V (July 2008). "The cullin 4B-based UV-damaged DNA-binding protein ligase binds to UV-damaged chromatin and ubiquitinates histone H2A". Cancer Res. 68 (13): 5014–22. doi:10.1158/0008-5472.CAN-07-6162. PMID 18593899.
- Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A (September 2008). "PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex". Genes Dev. 22 (18): 2496–506. doi:10.1101/gad.1676108. PMC 2546691. PMID 18794347.
Further reading
- Chu G, Chang E (1988). "Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA". Science. 242 (4878): 564–7. doi:10.1126/science.3175673. PMID 3175673.
- Lee TH, Elledge SJ, Butel JS (1995). "Hepatitis B virus X protein interacts with a probable cellular DNA repair protein". J. Virol. 69 (2): 1107–14. PMC 188683. PMID 7815490.
- Keeney S, Eker AP, Brody T, et al. (1994). "Correction of the DNA repair defect in xeroderma pigmentosum group E by injection of a DNA damage-binding protein" (PDF). Proc. Natl. Acad. Sci. U.S.A. 91 (9): 4053–6. doi:10.1073/pnas.91.9.4053. PMC 43721. PMID 8171034.
- Keeney S, Chang GJ, Linn S (1993). "Characterization of a human DNA damage binding protein implicated in xeroderma pigmentosum E.". J. Biol. Chem. 268 (28): 21293–300. PMID 8407967.
- Hwang BJ, Liao JC, Chu G (1996). "Isolation of a cDNA encoding a UV-damaged DNA binding factor defective in xeroderma pigmentosum group E cells". Mutat. Res. 362 (1): 105–17. doi:10.1016/0921-8777(95)00040-2. PMID 8538642.
- Nichols AF, Ong P, Linn S (1996). "Mutations specific to the xeroderma pigmentosum group E Ddb- phenotype". J. Biol. Chem. 271 (40): 24317–20. doi:10.1074/jbc.271.40.24317. PMID 8798680.
- Liu W, Nichols AF, Graham JA, et al. (2000). "Nuclear transport of human DDB protein induced by ultraviolet light". J. Biol. Chem. 275 (28): 21429–34. doi:10.1074/jbc.M000961200. PMID 10777491.
- Martinez E, Palhan VB, Tjernberg A, et al. (2001). "Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo". Mol. Cell. Biol. 21 (20): 6782–95. doi:10.1128/MCB.21.20.6782-6795.2001. PMC 99856. PMID 11564863.
- Chen X, Zhang Y, Douglas L, Zhou P (2002). "UV-damaged DNA-binding proteins are targets of CUL-4A-mediated ubiquitination and degradation". J. Biol. Chem. 276 (51): 48175–82. doi:10.1074/jbc.M106808200. PMID 11673459.
- Rapić-Otrin V, McLenigan MP, Bisi DC, et al. (2002). "Sequential binding of UV DNA damage binding factor and degradation of the p48 subunit as early events after UV irradiation". Nucleic Acids Res. 30 (11): 2588–98. doi:10.1093/nar/30.11.2588. PMC 117178. PMID 12034848.
- Bergametti F, Sitterlin D, Transy C (2002). "Turnover of hepatitis B virus X protein is regulated by damaged DNA-binding complex". J. Virol. 76 (13): 6495–501. doi:10.1128/JVI.76.13.6495-6501.2002. PMC 136256. PMID 12050362.
- Bontron S, Lin-Marq N, Strubin M (2002). "Hepatitis B virus X protein associated with UV-DDB1 induces cell death in the nucleus and is functionally antagonized by UV-DDB2". J. Biol. Chem. 277 (41): 38847–54. doi:10.1074/jbc.M205722200. PMID 12151405.
- Andrejeva J, Poole E, Young DF, et al. (2002). "The p127 subunit (DDB1) of the UV-DNA damage repair binding protein is essential for the targeted degradation of STAT1 by the V protein of the paramyxovirus simian virus 5". J. Virol. 76 (22): 11379–86. doi:10.1128/JVI.76.22.11379-11386.2002. PMC 136798. PMID 12388698.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Groisman R, Polanowska J, Kuraoka I, et al. (2003). "The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage". Cell. 113 (3): 357–67. doi:10.1016/S0092-8674(03)00316-7. PMID 12732143.
- Leupin O, Bontron S, Strubin M (2003). "Hepatitis B virus X protein and simian virus 5 V protein exhibit similar UV-DDB1 binding properties to mediate distinct activities". J. Virol. 77 (11): 6274–83. doi:10.1128/JVI.77.11.6274-6283.2003. PMC 154990. PMID 12743284.
- Wertz IE, O'Rourke KM, Zhang Z, et al. (2004). "Human De-etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase" (PDF). Science. 303 (5662): 1371–4. doi:10.1126/science.1093549. PMID 14739464.
- Bouwmeester T, Bauch A, Ruffner H, et al. (2004). "A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway". Nat. Cell Biol. 6 (2): 97–105. doi:10.1038/ncb1086. PMID 14743216.