FANCG
Fanconi anemia group G protein is a protein that in humans is encoded by the FANCG gene.[5][6][7]
Function
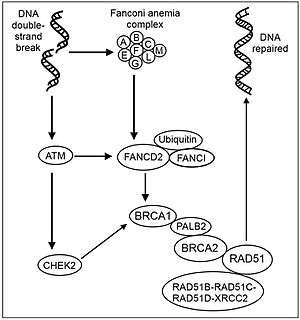
FANCG, involved in Fanconi anemia, confers resistance to both hygromycin B and mitomycin C. FANCG contains a 5-prime GC-rich untranslated region characteristic of housekeeping genes. The putative 622-amino acid protein has a leucine-zipper motif at its N-terminus. Fanconi anemia is an autosomal recessive disorder with diverse clinical symptoms, including developmental anomalies, bone marrow failure, and early occurrence of malignancies. A minimum of 8 FA genes have been identified. The FANCG gene is responsible for complementation group G.[7]
The clinical phenotype of all Fanconi anemia (FA) complementation groups is similar. This phenotype is characterized by progressive bone marrow failure, cancer proneness and typical birth defects. The main cellular phenotype is hypersensitivity to DNA damage, particularly inter-strand DNA crosslinks. The FA proteins interact through a multiprotein pathway. DNA interstrand crosslinks are highly deleterious damages that are repaired by homologous recombination involving coordination of FA proteins and breast cancer susceptibility gene 1 (BRCA1), but the exact biochemical roles of these proteins is currently unclear.
A nuclear complex containing FANCG (as well as FANCA, FANCB, FANCC, FANCE, FANCF, FANCL and FANCM) is essential for the activation of the FANCD2 protein to the mono-ubiquitinated isoform.[8] In normal, non-mutant, cells FANCD2 is mono-ubiquinated in response to DNA damage. Activated FANCD2 protein co-localizes with BRCA1 (breast cancer susceptibility protein) at ionizing radiation-induced foci and in synaptonemal complexes of meiotic chromosomes (see Figure: Recombinational repair of double strand damage).
Meiosis
Activated FANCD2 protein may function prior to the initiation of meiotic recombination, perhaps to prepare chromosomes for synapsis, or to regulate subsequent recombination events.[15]
Male and female FANCG mutant mice have defective gametogenesis, hypogonadism and impaired fertility, consistent with the phenotype of FA patients.[16][17] In the non-mutant mouse, FANCG protein is expressed in spermatogonia, preleptotene spermatocytes and spermatocytes in the leptotene, zygotene and early pachytene stages of meiosis.[18]
Aging
Loss of FANCG causes neural progenitor apoptosis during forebrain development, likely related to defective DNA repair.[19] (Sii-Felice et al., 2008). This effect persists in adulthood leading to depletion of the neural stem cell pool with aging. The FA phenotype can be interpreted as a premature aging of stem cells, DNA damages being the driving force of aging.[19] (Also see DNA damage theory of aging).
Interactions
FANCG has been shown to interact with FANCF,[20][21][22][23]
FANCA,[22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39] FANCE[23][37][40] and BRCA2.[41]
References
- GRCh38: Ensembl release 89: ENSG00000221829 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000028453 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Liu N, Lamerdin JE, Tucker JD, Zhou ZQ, Walter CA, Albala JS, Busch DB, Thompson LH (Sep 1997). "The human XRCC9 gene corrects chromosomal instability and mutagen sensitivities in CHO UV40 cells". Proc Natl Acad Sci U S A. 94 (17): 9232–7. doi:10.1073/pnas.94.17.9232. PMC 23130. PMID 9256465.
- Joenje H, Oostra AB, Wijker M, di Summa FM, van Berkel CG, Rooimans MA, Ebell W, van Weel M, Pronk JC, Buchwald M, Arwert F (Nov 1997). "Evidence for at least eight Fanconi anemia genes". Am J Hum Genet. 61 (4): 940–4. doi:10.1086/514881. PMC 1715980. PMID 9382107.
- "Entrez Gene: FANCG Fanconi anemia, complementation group G".
- D'Andrea AD (2010). "Susceptibility pathways in Fanconi's anemia and breast cancer". N. Engl. J. Med. 362 (20): 1909–19. doi:10.1056/NEJMra0809889. PMC 3069698. PMID 20484397.
- Sobeck A, Stone S, Landais I, de Graaf B, Hoatlin ME (2009). "The Fanconi anemia protein FANCM is controlled by FANCD2 and the ATR/ATM pathways". J. Biol. Chem. 284 (38): 25560–8. doi:10.1074/jbc.M109.007690. PMC 2757957. PMID 19633289.
- Castillo P, Bogliolo M, Surralles J (2011). "Coordinated action of the Fanconi anemia and ataxia telangiectasia pathways in response to oxidative damage". DNA Repair (Amst.). 10 (5): 518–25. doi:10.1016/j.dnarep.2011.02.007. PMID 21466974.
- Stolz A, Ertych N, Bastians H (2011). "Tumor suppressor CHK2: regulator of DNA damage response and mediator of chromosomal stability". Clin. Cancer Res. 17 (3): 401–5. doi:10.1158/1078-0432.CCR-10-1215. PMID 21088254.
- Taniguchi T, Garcia-Higuera I, Andreassen PR, Gregory RC, Grompe M, D'Andrea AD (2002). "S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51". Blood. 100 (7): 2414–20. doi:10.1182/blood-2002-01-0278. PMID 12239151.
- Park JY, Zhang F, Andreassen PR (2014). "PALB2: the hub of a network of tumor suppressors involved in DNA damage responses". Biochim. Biophys. Acta. 1846 (1): 263–75. doi:10.1016/j.bbcan.2014.06.003. PMC 4183126. PMID 24998779.
- Chun J, Buechelmaier ES, Powell SN (2013). "Rad51 paralog complexes BCDX2 and CX3 act at different stages in the BRCA1-BRCA2-dependent homologous recombination pathway". Mol. Cell. Biol. 33 (2): 387–95. doi:10.1128/MCB.00465-12. PMC 3554112. PMID 23149936.
- Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D'Andrea AD (2001). "Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway". Mol. Cell. 7 (2): 249–62. doi:10.1016/s1097-2765(01)00173-3. PMID 11239454.
- Yang Y, Kuang Y, Montes De Oca R, Hays T, Moreau L, Lu N, Seed B, D'Andrea AD (2001). "Targeted disruption of the murine Fanconi anemia gene, Fancg/Xrcc9". Blood. 98 (12): 3435–40. doi:10.1182/blood.v98.12.3435. PMID 11719385.
- Koomen M, Cheng NC, van de Vrugt HJ, Godthelp BC, van der Valk MA, Oostra AB, Zdzienicka MZ, Joenje H, Arwert F (2002). "Reduced fertility and hypersensitivity to mitomycin C characterize Fancg/Xrcc9 null mice". Hum. Mol. Genet. 11 (3): 273–81. doi:10.1093/hmg/11.3.273. PMID 11823446.
- Jamsai D, O'Connor AE, O'Donnell L, Lo JC, O'Bryan MK (2015). "Uncoupling of transcription and translation of Fanconi anemia (FANC) complex proteins during spermatogenesis". Spermatogenesis. 5 (1): e979061. doi:10.4161/21565562.2014.979061. PMC 4581071. PMID 26413409.
- Sii-Felice K, Barroca V, Etienne O, Riou L, Hoffschir F, Fouchet P, Boussin FD, Mouthon MA (2008). "Role of Fanconi DNA repair pathway in neural stem cell homeostasis". Cell Cycle. 7 (13): 1911–5. doi:10.4161/cc.7.13.6235. PMID 18604174.
- Léveillé F, Blom E, Medhurst AL, Bier P, Laghmani el H, Johnson M, Rooimans MA, Sobeck A, Waisfisz Q, Arwert F, Patel KJ, Hoatlin ME, Joenje H, de Winter JP (September 2004). "The Fanconi anemia gene product FANCF is a flexible adaptor protein" (PDF). J. Biol. Chem. 279 (38): 39421–30. doi:10.1074/jbc.M407034200. PMID 15262960.
- de Winter JP, van der Weel L, de Groot J, Stone S, Waisfisz Q, Arwert F, Scheper RJ, Kruyt FA, Hoatlin ME, Joenje H (November 2000). "The Fanconi anemia protein FANCF forms a nuclear complex with FANCA, FANCC and FANCG". Hum. Mol. Genet. 9 (18): 2665–74. doi:10.1093/hmg/9.18.2665. PMID 11063725.
- Gordon SM, Buchwald M (July 2003). "Fanconi anemia protein complex: mapping protein interactions in the yeast 2- and 3-hybrid systems". Blood. 102 (1): 136–41. doi:10.1182/blood-2002-11-3517. PMID 12649160.
- Medhurst AL, Huber PA, Waisfisz Q, de Winter JP, Mathew CG (February 2001). "Direct interactions of the five known Fanconi anaemia proteins suggest a common functional pathway". Hum. Mol. Genet. 10 (4): 423–9. doi:10.1093/hmg/10.4.423. PMID 11157805.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (October 2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. doi:10.1038/nature04209. PMID 16189514.
- Garcia-Higuera I, Kuang Y, Näf D, Wasik J, D'Andrea AD (July 1999). "Fanconi anemia proteins FANCA, FANCC, and FANCG/XRCC9 interact in a functional nuclear complex". Mol. Cell. Biol. 19 (7): 4866–73. doi:10.1128/mcb.19.7.4866. PMC 84285. PMID 10373536.
- Park SJ, Ciccone SL, Beck BD, Hwang B, Freie B, Clapp DW, Lee SH (July 2004). "Oxidative stress/damage induces multimerization and interaction of Fanconi anemia proteins". J. Biol. Chem. 279 (29): 30053–9. doi:10.1074/jbc.M403527200. PMID 15138265.
- van de Vrugt HJ, Koomen M, Berns MA, de Vries Y, Rooimans MA, van der Weel L, Blom E, de Groot J, Schepers RJ, Stone S, Hoatlin ME, Cheng NC, Joenje H, Arwert F (March 2002). "Characterization, expression and complex formation of the murine Fanconi anaemia gene product Fancg". Genes Cells. 7 (3): 333–42. doi:10.1046/j.1365-2443.2002.00518.x. PMID 11918676.
- Yagasaki H, Adachi D, Oda T, Garcia-Higuera I, Tetteh N, D'Andrea AD, Futaki M, Asano S, Yamashita T (December 2001). "A cytoplasmic serine protein kinase binds and may regulate the Fanconi anemia protein FANCA". Blood. 98 (13): 3650–7. doi:10.1182/blood.V98.13.3650. PMID 11739169.
- Huber PA, Medhurst AL, Youssoufian H, Mathew CG (February 2000). "Investigation of Fanconi anemia protein interactions by yeast two-hybrid analysis". Biochem. Biophys. Res. Commun. 268 (1): 73–7. doi:10.1006/bbrc.1999.2055. PMID 10652215.
- Kruyt FA, Abou-Zahr F, Mok H, Youssoufian H (November 1999). "Resistance to mitomycin C requires direct interaction between the Fanconi anemia proteins FANCA and FANCG in the nucleus through an arginine-rich domain". J. Biol. Chem. 274 (48): 34212–8. doi:10.1074/jbc.274.48.34212. PMID 10567393.
- Reuter T, Herterich S, Bernhard O, Hoehn H, Gross HJ (January 2000). "Strong FANCA/FANCG but weak FANCA/FANCC interaction in the yeast 2-hybrid system". Blood. 95 (2): 719–20. doi:10.1182/blood.V95.2.719. PMID 10627486.
- Blom E, van de Vrugt HJ, de Vries Y, de Winter JP, Arwert F, Joenje H (January 2004). "Multiple TPR motifs characterize the Fanconi anemia FANCG protein". DNA Repair (Amst.). 3 (1): 77–84. doi:10.1016/j.dnarep.2003.09.007. PMID 14697762.
- Kuang Y, Garcia-Higuera I, Moran A, Mondoux M, Digweed M, D'Andrea AD (September 2000). "Carboxy terminal region of the Fanconi anemia protein, FANCG/XRCC9, is required for functional activity". Blood. 96 (5): 1625–32. doi:10.1182/blood.V96.5.1625. PMID 10961856.
- Thomashevski A, High AA, Drozd M, Shabanowitz J, Hunt DF, Grant PA, Kupfer GM (June 2004). "The Fanconi anemia core complex forms four complexes of different sizes in different subcellular compartments". J. Biol. Chem. 279 (25): 26201–9. doi:10.1074/jbc.M400091200. PMID 15082718.
- Waisfisz Q, de Winter JP, Kruyt FA, de Groot J, van der Weel L, Dijkmans LM, Zhi Y, Arwert F, Scheper RJ, Youssoufian H, Hoatlin ME, Joenje H (August 1999). "A physical complex of the Fanconi anemia proteins FANCG/XRCC9 and FANCA". Proc. Natl. Acad. Sci. U.S.A. 96 (18): 10320–5. doi:10.1073/pnas.96.18.10320. PMC 17886. PMID 10468606.
- Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, Oostra AB, Yan Z, Ling C, Bishop CE, Hoatlin ME, Joenje H, Wang W (October 2003). "A novel ubiquitin ligase is deficient in Fanconi anemia". Nat. Genet. 35 (2): 165–70. doi:10.1038/ng1241. PMID 12973351.
- Taniguchi T, D'Andrea AD (October 2002). "The Fanconi anemia protein, FANCE, promotes the nuclear accumulation of FANCC". Blood. 100 (7): 2457–62. doi:10.1182/blood-2002-03-0860. PMID 12239156.
- Otsuki T, Young DB, Sasaki DT, Pando MP, Li J, Manning A, Hoekstra M, Hoatlin ME, Mercurio F, Liu JM (2002). "Fanconi anemia protein complex is a novel target of the IKK signalsome". J. Cell. Biochem. 86 (4): 613–23. doi:10.1002/jcb.10270. PMID 12210728.
- Garcia-Higuera I, Kuang Y, Denham J, D'Andrea AD (November 2000). "The fanconi anemia proteins FANCA and FANCG stabilize each other and promote the nuclear accumulation of the Fanconi anemia complex". Blood. 96 (9): 3224–30. doi:10.1182/blood.V96.9.3224. PMID 11050007.
- Pace P, Johnson M, Tan WM, Mosedale G, Sng C, Hoatlin M, de Winter J, Joenje H, Gergely F, Patel KJ (July 2002). "FANCE: the link between Fanconi anaemia complex assembly and activity". EMBO J. 21 (13): 3414–23. doi:10.1093/emboj/cdf355. PMC 125396. PMID 12093742.
- Hussain S, Witt E, Huber PA, Medhurst AL, Ashworth A, Mathew CG (October 2003). "Direct interaction of the Fanconi anaemia protein FANCG with BRCA2/FANCD1". Hum. Mol. Genet. 12 (19): 2503–10. doi:10.1093/hmg/ddg266. PMID 12915460.
Further reading
- de Winter JP, Waisfisz Q, Rooimans MA, van Berkel CG, Bosnoyan-Collins L, Alon N, et al. (November 1998). "The Fanconi anaemia group G gene FANCG is identical with XRCC9". Nat. Genet. 20 (3): 281–3. doi:10.1038/3093. PMID 9806548.
- Jelesko JG, Harper R, Furuya M, Gruissem W (August 1999). "Rare germinal unequal crossing-over leading to recombinant gene formation and gene duplication in Arabidopsis thaliana". Proc. Natl. Acad. Sci. U.S.A. 96 (18): 10302–7. doi:10.1073/pnas.96.18.10302. PMC 17883. PMID 10468603.
- Yamada T, Tachibana A, Shimizu T, et al. (2000). "Novel mutations of the FANCG gene causing alternative splicing in Japanese Fanconi anemia". J. Hum. Genet. 45 (3): 159–66. doi:10.1007/s100380050203. PMID 10807541.
- Demuth I, Wlodarski M, Tipping AJ, et al. (2000). "Spectrum of mutations in the Fanconi anaemia group G gene, FANCG/XRCC9". Eur. J. Hum. Genet. 8 (11): 861–8. doi:10.1038/sj.ejhg.5200552. PMID 11093276.
- McMahon LW, Sangerman J, Goodman SR, et al. (2001). "Human alpha spectrin II and the FANCA, FANCC, and FANCG proteins bind to DNA containing psoralen interstrand cross-links". Biochemistry. 40 (24): 7025–34. doi:10.1021/bi002917g. PMID 11401546.
- Futaki M, Igarashi T, Watanabe S, et al. (2002). "The FANCG Fanconi anemia protein interacts with CYP2E1: possible role in protection against oxidative DNA damage". Carcinogenesis. 23 (1): 67–72. doi:10.1093/carcin/23.1.67. PMID 11756225.