Nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter.[1][2] The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions.[3] At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead.
| Part of a series of articles on |
| Nanomaterials |
|---|
 |
| Carbon nanotubes |
| Fullerenes |
|
| Other nanoparticles |
| Nanostructured materials |
|
Nanoparticles are usually distinguished from microparticles (1-1000 µm), "fine particles" (sized between 100 and 2500 nm), and "coarse particles" (ranging from 2500 to 10,000 nm), because their smaller size drives very different physical or chemical properties, like colloidal properties and optical or electric properties.
Being more subject to the brownian motion, they usually do not sediment, like colloidal particles that conversely are usually understood to range from 1 to 1000 nm.
Being much smaller than the wavelengths of visible light (400-700 nm), nanoparticles cannot be seen with ordinary optical microscopes, requiring the use of electron microscopes. For the same reason, dispersions of nanoparticles in transparent media can be transparent,[4] whereas suspensions of larger particles usually scatter some or all visible light incident on them. Nanoparticles also easily pass through common filters, such as common ceramic candles,[5] so that separation from liquids requires special nanofiltration techniques.
The properties of nanoparticles often differ markedly from those of larger particles of the same substance. Since the typical diameter of an atom is between 0.15 and 0.6 nm, a large fraction of the nanoparticle's material lies within a few atomic diameters from its surface. Therefore, the properties of that surface layer may dominate over those of the bulk material. This effect is particularly strong for nanoparticles dispersed in a medium of different composition since the interactions between the two materials at their interface also becomes significant.[6]
Nanoparticles occur widely in nature and are objects of study in many sciences such as chemistry, physics, geology and biology. Being at the transition between bulk materials and atomic or molecular structures, they often exhibit phenomena that are not observed at either scale. They are an important component of atmospheric pollution, and key ingredients in many industrialized products such as paints, plastics, metals, ceramics, and magnetic articles. The production of nanoparticles with specific properties is an important branch of nanotechnology.
In general, the small size of nanoparticles leads to a lower concentration of point defects compared to their bulk counterparts,[7] but they do support a variety of dislocations that can be visualized using high-resolution electron microscopes.[8] However, nanoparticles exhibit different dislocation mechanics, which, together with their unique surface structures, results in mechanical properties that are different from the bulk material.[9][10][11]
Anisotropy in a nanoparticle leads to a lot of changes in the properties of the nanoparticles. Non-spherical nanoparticles of gold, silver, and platinum due to their fascinating optical properties are finding diverse applications and are of great interest in the field of research. Non-spherical geometries of nanoprisms give rise to high effective cross-sections and deeper colors of the colloidal solutions.[12] The possibility of shifting the resonance wavelengths by tuning the particle geometry is very interesting for using these nanoparticles in the fields of molecular labeling, for biomolecular assays, trace metal detection, and nanotechnical applications. Anisotropic nanoparticles display a specific absorption behavior and stochastic particle orientation under unpolarized light, showing a distinct resonance mode for each excitable axis . This property can be explained based on the fact that on a daily basis there are new developments being made in the field of synthesis of these nanoparticles for preparing them in high yield.[12]
Definitions
IUPAC
In its 2012 proposed terminology for biologically related polymers, the IUPAC defined a nanoparticle as "a particle of any shape with dimensions in the 1 × 10−9 and 1 × 10−7 m range".[2] This definition evolved from one given by IUPAC in 1997.[13][14]
In another 2012 publication, the IUPAC extends the term to include tubes and fibers with only two dimensions below 100 nm.[3]
ISO
According to the International Standards Organization (ISO) technical specification 80004, a nanoparticle is an object with all three external dimensions in the nanoscale, whose longest and shortest axes do not differ significantly, with a significant difference typically being a factor of at least 3.[15]
Common usage
"Nanoscale" is usually understood to be the range from 1 to 100 nm because the novel properties that differentiate particles from the bulk material typically develop at that range of sizes.
For some properties, like transparency or turbidity, ultrafiltration, stable dispersion, etc., substantial changes characteristic of nanoparticles are observed for particles as large as 500 nm. Therefore, the term is sometimes extended to that size range.
Related concepts
Nanoclusters are agglomerates of nanoparticles with at least one dimension between 1 and 10 nanometers and a narrow size distribution. Nanopowders[16] are agglomerates of ultrafine particles, nanoparticles, or nanoclusters. Nanometer-sized single crystals, or single-domain ultrafine particles, are often referred to as nanocrystals.
The terms colloid and nanoparticle are not interchangeable. A colloid is a mixture which has particles of one phase dispersed or suspended within an other phase. The term applies only if the particles are larger than atomic dimensions but small enough to exhibit Brownian motion, with the critical size range (or particle diameter) typically ranging from nanometers (10−9 m) to micrometers (10−6 m).[17] Colloids can contain particles too large to be nanoparticles, and nanoparticles can exist in non-colloidal form, for examples as a powder or in a solid matrix.
History
Natural occurrence
Nanoparticles are naturally produced by many cosmological,[18] geological,[18][19] meteorological, and biological processes. A significant fraction (by number, if not by mass) of interplanetary dust, that is still falling on the Earth at the rate of thousands of tons per year, is in the nanoparticle range;[20][21] and the same is true of atmospheric dust particles. Many viruses have diameters in the nanoparticle range.
Pre-industrial technology
Nanoparticles were used by artisans since prehistory, albeit without knowledge of their nature. They were used by glassmakers and potters in Classical Antiquity, as exemplified by the Roman Lycurgus cup of dichroic glass (4th century CE) and the lusterware pottery of Mesopotamia (9th century CE).[22][23][24] The latter is characterized by silver and copper nanoparticles dispersed in the glassy glaze.
19th century
Michael Faraday provided the first description, in scientific terms, of the optical properties of nanometer-scale metals in his classic 1857 paper. In a subsequent paper, the author (Turner) points out that: "It is well known that when thin leaves of gold or silver are mounted upon glass and heated to a temperature that is well below a red heat (~500 °C), a remarkable change of properties takes place, whereby the continuity of the metallic film is destroyed. The result is that white light is now freely transmitted, reflection is correspondingly diminished, while the electrical resistivity is enormously increased."[25][26][27]
20th century
During the 1970s and 80s, when the first thorough fundamental studies with nanoparticles were underway in the United States (by Granqvist and Buhrman)[28] and Japan (within an ERATO Project),[29] researchers used the term ultrafine particles. However, during the 1990s, before the National Nanotechnology Initiative was launched in the United States, the term nanoparticle had become more common (for example, see the same senior author's paper 20 years later addressing the same issue, lognormal distribution of sizes[30]).
Morphology and structure
Nanoparticles occur in a great variety of shapes, which have been given many informal names such as nanospheres,[31] nanorods, nanochains,[32] nanostars, nanoflowers, nanoreefs,[33] nanowhiskers, nanofibers, and nanoboxes.[34]
The shapes of nanoparticles may be determined by the intrinsic crystal habit of the material, or by the influence of the environment around their creation, such as the inhibition of crystal growth on certain faces by coating additives, the shape of emulsion droplets and micelles in the precursor preparation, or the shape of pores in a surrounding solid matrix.[35] Some applications of nanoparticles may require specific shapes, as well as specific sizes or size ranges.
Amorphous particles typically adopt a spherical shape (due to their microstructural isotropy).
The study of fine particles is called micromeritics.
Variations
Semi-solid and soft nanoparticles have been produced. A prototype nanoparticle of semi-solid nature is the liposome. Various types of liposome nanoparticles are currently used clinically as delivery systems for anticancer drugs and vaccines.
The breakdown of biopolymers into their nanoscale building blocks is considered a potential route to produce nanoparticles with enhanced biocompatibility and biodegradability. The most common example is the production of nanocellulose from wood pulp.[36] Other examples are nanolignin, nanchitin, or nanostarches.[37]
Nanoparticles with one half hydrophilic and the other half hydrophobic are termed Janus particles and are particularly effective for stabilizing emulsions. They can self-assemble at water/oil interfaces and act as Pickering stabilizers.
Hydrogel nanoparticles made of N-isopropylacrylamide hydrogel core shell can be dyed with affinity baits, internally.[38] These affinity baits allow the nanoparticles to isolate and remove undesirable proteins while enhancing the target analytes.[38]
Properties

The properties of a material in nanoparticle form are usually very different from those of the bulk material even when divided into micrometer-size particles.[39][40][41] A number of causes contribute to that effect.
Large area/volume ratio
A bulk material should have constant physical properties (such as thermal and electrical conductivity, stiffness, density, and viscosity) regardless of its size. However, in a nanoparticle, the volume of the surface layer (the material that is within a few atomic diameters of the surface) becomes a significant fraction of the particle's volume; whereas that fraction is insignificant for particles with diameter of one micrometer or more.
Interfacial layer
For nanoparticles dispersed in a medium of different composition, the interfacial layer — formed by ions and molecules from the medium that are within a few atomic diameters of the surface of each particle — can mask or change its chemical and physical properties. Indeed, that layer can be considered an integral part of each nanoparticle.[6]
Solvent affinity
Suspensions of nanoparticles are possible since the interaction of the particle surface with the solvent is strong enough to overcome density differences, which otherwise usually result in a material either sinking or floating in a liquid.
Coatings
_with_complete_passivation.png)
Nanoparticles often develop or receive coatings of other substances, distinct from both the particle's material and of the surrounding medium. Even when only a single molecule thick, these coatings can radically change the particles' properties, such as and chemical reactivity, catalytic activity, and stability in suspension.
Diffusion across the surface
The high surface area of a material in nanoparticle form allows heat, molecules, and ions to diffuse into or out of the particles at very large rates. The small particle diameter, on the other hand, allows the whole material to reach homogeneous equilibrium with respect to diffusion in a very short time. Thus many processes that depend on diffusion, such as sintering can take place at lower temperatures and over shorter time scales.
Ferromagnetic and ferroelectric effects
The small size of nanoparticles affects their magnetic and electric properties. For example, while particles of ferromagnetic materials in the micrometer range are widely used in magnetic recording media, for the stability of their magnetization state, those smaller than 10 nm can change their state as the result of thermal energy at ordinary temperatures, thus making them unsuitable for that application.[42]
Mechanical Properties
The reduced vacancy concentration in nanocrystals can negatively affect the motion of dislocations, since dislocation climb requires vacancy migration. In addition, there exists a very high internal pressure due to the surface stress present in small nanoparticles with high radii of curvature.[43] This causes a lattice strain that is inversely proportional to the size of the particle,[44] also well-known to impede dislocation motion, in the same way as it does in the work hardening of materials.[45] For example, gold nanoparticles are significantly harder than the bulk material.[46] Furthermore, the high surfact-to-volume ratio in nanoparticles makes dislocations more likely to interact with the particle surface. In particular, this affects the nature of the dislocation source and allows the dislocations to escape the particle before they can multiply, reducing the dislocation density and thus the extent of plastic deformation.[47][48]
There are unique challenges associated with the measurement of mechanical properties on the nanoscale, as conventional means such as the universal testing machine cannot be employed. As a result, new techniques such as nanoindentation have been developed that complement existing electron microscope and scanning probe methods.[49]
Melting point depression
A material may have lower melting point in nanoparticle form than in the bulk form. For example, 2.5 nm gold nanoparticles melt at about 300 °C, whereas bulk gold melts at 1064 °C.[50]
Quantum mechanics effects
Quantum mechanics effects become noticeable for nanoscale objects.[51] They include quantum confinement in semiconductor particles, localized surface plasmons[51] in some metal particles, and superparamagnetism in magnetic materials. Quantum dots are nanoparticles of semiconducting material that are small enough (typically sub 10 nm or less) to have quantized electronic energy levels.
Quantum effects are responsible for the deep-red to black color of gold or silicon nanopowders and nanoparticle suspensions.[50] Absorption of solar radiation is much higher in materials composed of nanoparticles than in thin films of continuous sheets of material. In both solar PV and solar thermal applications, by controlling the size, shape, and material of the particles, it is possible to control solar absorption.[52][53][54][55]
Core-shell nanoparticles can support simultaneously both electric and magnetic resonances, demonstrating entirely new properties when compared with bare metallic nanoparticles if the resonances are properly engineered.[56][57][58] The formation of the core-shell structure from two different metals enables an energy exchange between the core and the shell, typically found in upconverting nanoparticles and downconverting nanoparticles, and causes a shift in the emission wavelength spectrum.[59]
By introducing a dielectric layer, plasmonic core (metal)-shell (dielectric) nanoparticles enhance light absorption by increasing scattering. Recently, the metal core-dielectric shell nanoparticle has demonstrated a zero backward scattering with enhanced forward scattering on a silicon substrate when surface plasmon is located in front of a solar cell.[60]
Regular packing
Nanoparticles of sufficiently uniform size may spontaneously settle into regular arrangements, forming a colloidal crystal. These arrangements may exhibit original physical properties, such as observed in photonic crystals[61][62]
Production
Artificial nanoparticles can be created from any solid or liquid material, including metals, dielectrics, and semiconductors. They may be internally homogeneous or heterogenous, e.g. with a Core–shell structure.[56][57][58]
There are several methods for creating nanoparticles, including gas condensation, attrition, chemical precipitation,[63] ion implantation, pyrolysis and hydrothermal synthesis.
Mechanical
Friable macro- or micro-scale solid particles can be ground in a ball mill, a planetary ball mill, or other size-reducing mechanism until enough of them are in the nanoscale size range. The resulting powder can be air classified to extract the nanoparticles.[64][65][66]
Breakdown of biopolymers
Biopolymers like cellulose, lignin, chitin, or starch may be broken down into their individual nanoscale building blocks, obtaining anisotropic fiber- or needle-like nanoparticles. The biopolymers are disintegrated mechanically in combination with chemical oxidation or enzymatic treatment to promote breakup, or hydrolysed using acid.
Pyrolysis
Another method to create nanoparticles is to turn a suitable precursor substance, such as a gas or aerosol, into solid particles by combustion or pyrolysis. This is a generalization of the burning of hydrocarbons or other organic vapors to generate soot.
Traditional pyrolysis often results in aggregates and agglomerates rather than single primary particles. This inconvenience can be avoided by ultrasonic nozzle spray pyrolysis, in which the precursor liquid is forced through an orifice at high pressure.
Condensation from plasma
Nanoparticles of refractory materials, such as silica and other oxides, carbides, and nitrides, can be created by vaporizing the solid with a thermal plasma, which can reach temperatures of 10,000 kelvin, and then condensing the vapor by expansion or quenching in a suitable gas or liquid. The plasma can be produced by dc jet, electric arc, or radio frequency (RF) induction. Metal wires can be vaporized by the exploding wire method.
In RF induction plasma torches, energy coupling to the plasma is accomplished through the electromagnetic field generated by the induction coil. The plasma gas does not come in contact with electrodes, thus eliminating possible sources of contamination and allowing the operation of such plasma torches with a wide range of gases including inert, reducing, oxidizing, and other corrosive atmospheres. The working frequency is typically between 200 kHz and 40 MHz. Laboratory units run at power levels in the order of 30–50 kW, whereas the large-scale industrial units have been tested at power levels up to 1 MW. As the residence time of the injected feed droplets in the plasma is very short, it is important that the droplet sizes are small enough in order to obtain complete evaporation.
Inert gas condensation
Inert-gas condensation is frequently used to produce metallic nanoparticles. The metal is evaporated in a vacuum chamber containing a reduced atmosphere of an inert gas.[67] Condensation of the supersaturated metal vapor results in creation of nanometer-size particles, which can be entrained in the inert gas stream and deposited on a substrate or studied in situ. Early studies were based on thermal evaporation.[67] Using magnetron sputtering to create the metal vapor allows to achieve higher yields.[68] The method can easily be generalized to alloy nanoparticles by choosing appropriate metallic targets. The use of sequential growth schemes, where the particles travel through a second metallic vapor, results in growth of core-shell (CS) structures.[69][70][71]
Radiolysis method
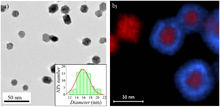
Nanoparticles can also be formed using radiation chemistry. Radiolysis from gamma rays can create strongly active free radicals in solution. This relatively simple technique uses a minimum number of chemicals. These including water, a soluble metallic salt, a radical scavenger (often a secondary alcohol), and a surfactant (organic capping agent). High gamma doses on the order of 104 Gray are required. In this process, reducing radicals will drop metallic ions down to the zero-valence state. A scavenger chemical will preferentially interact with oxidizing radicals to prevent the re-oxidation of the metal. Once in the zero-valence state, metal atoms begin to coalesce into particles. A chemical surfactant surrounds the particle during formation and regulates its growth. In sufficient concentrations, the surfactant molecules stay attached to the particle. This prevents it from dissociating or forming clusters with other particles. Formation of nanoparticles using the radiolysis method allows for tailoring of particle size and shape by adjusting precursor concentrations and gamma dose.[73]
Wet chemistry
Nanoparticles of certain materials can be created by "wet" chemical processes, in which solutions of suitable compounds are mixed or otherwise treated to form an insoluble precipitate of the desired material. The size of the particles of the latter is adjusted by choosing the concentration of the reagents and the temperature of the solutions, and through the addition of suitable inert agents that affect the viscosity and diffusion rate of the liquid. With different parameters, the same general process may yield other nanoscale structures of the same material, such as aerogels and other porous networks.[74]
The nanoparticles formed by this method are then separated from the solvent and soluble byproducts of the reaction by a combination of evaporation, sedimentation, centrifugation, washing, and filtration. Alternatively, if the particles are meant to be deposited on the surface of some solid substrate, the starting solutions can be by coated on that surface by dipping or spin-coating, and the reaction can be carried out in place.
The suspension of nanoparticles that result from this process is an example of colloid. Typical instances of this method are the production of metal oxide or hydroxide nanoparticles by hydrolysis of metal alkoxides and chlorides.[75][4]
Besides being cheap and convenient, the wet chemical approach allows fine control of the particle's chemical composition. Even small quantities of dopants, such as organic dyes and rare earth metals, can be introduced in the reagent solutions end up uniformly dispersed in the final product.[76][77]
Ion implantation
Ion implantation may be used to treat the surfaces of dielectric materials such as sapphire and silica to make composites with near-surface dispersions of metal or oxide nanoparticles.
Functionalization
Many properties of nanoparticles, notably stability, solubility, and chemical or biological activity, can be radically altered by coating them with various substances — a process called functionalization. Functionalized nanomaterial-based catalysts can be used for catalysis of many known organic reactions.
For example, suspensions of graphene particles can be stabilized by functionalization with gallic acid groups.[78]
For biological applications, the surface coating should be polar to give high aqueous solubility and prevent nanoparticle aggregation. In serum or on the cell surface, highly charged coatings promote non-specific binding, whereas polyethylene glycol linked to terminal hydroxyl or methoxy groups repel non-specific interactions.[79][80]
Nanoparticles can be linked to biological molecules that can act as address tags, directing them to specific sites within the body[81] specific organelles within the cell,[82] or causing them to follow specifically the movement of individual protein or RNA molecules in living cells.[83] Common address tags are monoclonal antibodies, aptamers, streptavidin or peptides. These targeting agents should ideally be covalently linked to the nanoparticle and should be present in a controlled number per nanoparticle. Multivalent nanoparticles, bearing multiple targeting groups, can cluster receptors, which can activate cellular signaling pathways, and give stronger anchoring. Monovalent nanoparticles, bearing a single binding site,[84][85][86] avoid clustering and so are preferable for tracking the behavior of individual proteins.
Coatings that mimic those of red blood cells can help nanoparticles evade the immune system.[87]
Uniformity requirements
The chemical processing and synthesis of high-performance technological components for the private, industrial, and military sectors requires the use of high-purity ceramics (oxide ceramics, such as aluminium oxide or copper(II) oxide), polymers, glass-ceramics, and composite materials, as metal carbides (SiC), nitrides (Aluminum nitrides, Silicon nitride), metals (Al, Cu), non-metals (graphite, carbon nanotubes) and layered (Al + Aluminium carbonate, Cu + C). In condensed bodies formed from fine powders, the irregular particle sizes and shapes in a typical powder often lead to non-uniform packing morphologies that result in packing density variations in the powder compact.
Uncontrolled agglomeration of powders due to attractive van der Waals forces can also give rise to microstructural heterogeneity. Differential stresses that develop as a result of non-uniform drying shrinkage are directly related to the rate at which the solvent can be removed, and thus highly dependent upon the distribution of porosity. Such stresses have been associated with a plastic-to-brittle transition in consolidated bodies, and can yield to crack propagation in the unfired body if not relieved.[88][89][90]
In addition, any fluctuations in packing density in the compact as it is prepared for the kiln are often amplified during the sintering process, yielding inhomogeneous densification. Some pores and other structural defects associated with density variations have been shown to play a detrimental role in the sintering process by growing and thus limiting end-point densities. Differential stresses arising from inhomogeneous densification have also been shown to result in the propagation of internal cracks, thus becoming the strength-controlling flaws.[91][92][93]
Inert gas evaporation and inert gas deposition[28][29] are free many of these defects due to the distillation (cf. purification) nature of the process and having enough time to form single crystal particles, however even their non-aggreated deposits have lognormal size distribution, which is typical with nanoparticles.[29] The reason why modern gas evaporation techniques can produce a relatively narrow size distribution is that aggregation can be avoided.[29] However, even in this case, random residence times in the growth zone, due to the combination of drift and diffusion, result in a size distribution appearing lognormal.[30]
It would, therefore, appear desirable to process a material in such a way that it is physically uniform with regard to the distribution of components and porosity, rather than using particle size distributions that will maximize the green density. The containment of a uniformly dispersed assembly of strongly interacting particles in suspension requires total control over interparticle forces. Monodisperse nanoparticles and colloids provide this potential.[94]
Characterization
Nanoparticles have different analytical requirements than conventional chemicals, for which chemical composition and concentration are sufficient metrics. Nanoparticles have other physical properties that must be measured for a complete description, such as size, shape, surface properties, crystallinity, and dispersion state. Additionally, sampling and laboratory procedures can perturb their dispersion state or bias the distribution of other properties.[95][96] In environmental contexts, an additional challenge is that many methods cannot detect low concentrations of nanoparticles that may still have an adverse effect.[95] For some applications, nanoparticles may be characterized in complex matrices such as water, soil, food, polymers, inks, complex mixtures of organic liquids such as in cosmetics, or blood.[97][98]
There are several overall categories of methods used to characterize nanoparticles. Microscopy methods generate images of individual nanoparticles to characterize their shape, size, and location. Electron microscopy and scanning probe microscopy are the dominant methods. Because nanoparticles have a size below the diffraction limit of visible light, conventional optical microscopy is not useful. Electron microscopes can be coupled to spectroscopic methods that can perform elemental analysis. Microscopy methods are destructive and can be prone to undesirable artifacts from sample preparation, or from probe tip geometry in the case of scanning probe microscopy. Additionally, microscopy is based on single-particle measurements, meaning that large numbers of individual particles must be characterized to estimate their bulk properties.[95][97]
Spectroscopy, which measures the particles' interaction with electromagnetic radiation as a function of wavelength, is useful for some classes of nanoparticles to characterize concentration, size, and shape. X-ray, ultraviolet–visible, infrared, and nuclear magnetic resonance spectroscopy can be used with nanoparticles.[95][97] Light scattering methods using laser light, X-rays, or neutron scattering are used to determine particle size, with each method suitable for different size ranges and particle compositions.[95][97] Some miscellaneous methods are electrophoresis for surface charge, the Brunauer–Emmett–Teller method for surface area, and X-ray diffraction for crystal structure,[95] as well as mass spectrometry for particle mass, and particle counters for particle number.[97] Chromatography, centrifugation, and filtration techniques can be used to separate nanoparticles by size or other physical properties before or during characterization.[95]
Health and safety
Nanoparticles present possible dangers, both medically and environmentally.[99] [100] [101][102] Most of these are due to the high surface to volume ratio, which can make the particles very reactive or catalytic.[103] They are also able to pass through cell membranes in organisms, and their interactions with biological systems are relatively unknown.[104][105] However, it is unlikely the particles would enter the cell nucleus, Golgi complex, endoplasmic reticulum or other internal cellular components due to the particle size and intercellular agglomeration.[106] A recent study looking at the effects of ZnO nanoparticles on human immune cells has found varying levels of susceptibility to cytotoxicity.[107] There are concerns that pharmaceutical companies, seeking regulatory approval for nano-reformulations of existing medicines, are relying on safety data produced during clinical studies of the earlier, pre-reformulation version of the medicine. This could result in regulatory bodies, such as the FDA, missing new side effects that are specific to the nano-reformulation.[108] However considerable research has demonstrated that zinc nanoparticles are not absorbed into the bloodstream in vivo.[109]
Concern has also been raised over the health effects of respirable nanoparticles from certain combustion processes.[110][111] Preclinical investigations have demonstrated that some inhaled or injected noble metal nano-architectures avoid persistence in organisms.[112][113] As of 2013 the U.S. Environmental Protection Agency was investigating the safety of the following nanoparticles:[114]
- Carbon Nanotubes: Carbon materials have a wide range of uses, ranging from composites for use in vehicles and sports equipment to integrated circuits for electronic components. The interactions between nanomaterials such as carbon nanotubes and natural organic matter strongly influence both their aggregation and deposition, which strongly affects their transport, transformation, and exposure in aquatic environments. In past research, carbon nanotubes exhibited some toxicological impacts that will be evaluated in various environmental settings in current EPA chemical safety research. EPA research will provide data, models, test methods, and best practices to discover the acute health effects of carbon nanotubes and identify methods to predict them.[114]
- Cerium oxide: Nanoscale cerium oxide is used in electronics, biomedical supplies, energy, and fuel additives. Many applications of engineered cerium oxide nanoparticles naturally disperse themselves into the environment, which increases the risk of exposure. There is ongoing exposure to new diesel emissions using fuel additives containing CeO2 nanoparticles, and the environmental and public health impacts of this new technology are unknown. EPA's chemical safety research is assessing the environmental, ecological, and health implications of nanotechnology-enabled diesel fuel additives.[114]
- Titanium dioxide: Nano titanium dioxide is currently used in many products. Depending on the type of particle, it may be found in sunscreens, cosmetics, and paints and coatings. It is also being investigated for use in removing contaminants from drinking water.[114]
- Nano Silver: Nano silver is being incorporated into textiles, clothing, food packaging, and other materials to eliminate bacteria. EPA and the U.S. Consumer Product Safety Commission are studying certain products to see whether they transfer nano-size silver particles in real-world scenarios. EPA is researching this topic to better understand how much nano-silver children come in contact with in their environments.[114]
- Iron: While nano-scale iron is being investigated for many uses, including “smart fluids” for uses such as optics polishing and as a better-absorbed iron nutrient supplement, one of its more prominent current uses is to remove contamination from groundwater. This use, supported by EPA research, is being piloted at a number of sites across the United States.[114]
Regulation
As of 2016, the U.S. Environmental Protection Agency had conditionally registered, for a period of four years, only two nanomaterial pesticides as ingredients. The EPA differentiates nanoscale ingredients from non-nanoscale forms of the ingredient, but there is little scientific data about potential variation in toxicity. Testing protocols still need to be developed.[115]
Applications
As the most prevalent morphology of nanomaterials used in consumer products, nanoparticles have an enormous range of potential and actual applications. Table below summarizes the most common nanoparticles used in various product types available on the global markets.
Clay nanoparticles, when incorporated into polymer matrices, increase reinforcement, leading to stronger plastics, verifiable by a higher glass transition temperature and other mechanical property tests. These nanoparticles are hard, and impart their properties to the polymer (plastic). Nanoparticles have also been attached to textile fibers in order to create smart and functional clothing.[116]
The inclusion of nanoparticles in a solid or liquid medium can substantially change its mechanical properties, such as elasticity, plasticity, viscosity, compressibility, .[117][118]
Being smaller than the wavelengths of visible light, nanoparticles can be dispersed in transparent media without affecting its transparency at those wavelengths. This property is exploited in many applications, such as photocatalysis.[119]
Nanoscale particles are used in biomedical applications as drug carriers or imaging contrast agents.
Scientific research on nanoparticles is intense as they have many potential applications in medicine, physics,[121][122][123] optics,[124][125][126] and electronics.[57][53][51][54] The U.S. National Nanotechnology Initiative offers government funding focused on nanoparticle research.|The use of nanoparticles in laser dye-doped poly(methyl methacrylate) (PMMA) laser gain media was demonstrated in 2003 and it has been shown to improve conversion efficiencies and to decrease laser beam divergence.[127] Researchers attribute the reduction in beam divergence to improved dn/dT characteristics of the organic-inorganic dye-doped nanocomposite. The optimum composition reported by these researchers is 30% w/w of SiO2 (~ 12 nm) in dye-doped PMMA.|Nanoparticles are being investigated as potential drug delivery system.[128] Drugs, growth factors or other biomolecules can be conjugated to nano particles to aid targeted delivery.[129] This nanoparticle-assisted delivery allows for spatial and temporal controls of the loaded drugs to achieve the most desirable biological outcome. Nanoparticles are also studied for possible applications as dietary supplements for delivery of biologically active substances, for example mineral elements.[130] Bitumen modification through clay and fumed silica nanoparticles can be considered as an interesting low-cost technique in asphalt pavement engineering providing novel perspectives in making asphalt materials more durable.[131]
Nanoparticles have been found to impart some extra properties to various day to day products. For example, the presence of titanium dioxide nanoparticles imparts what is known as the self-cleaning effect, which lend useful water-repellant and antibacterial properties to paints and other products. Zinc oxide nanoparticles have been found to have superior UV blocking properties and are widely used in the preparation of sunscreen lotions,[132] being completely photostable[133] though toxic.[134][135][136][137][138][139]
See also
- Ceramic engineering
- Coating
- Colloid
- Colloid-facilitated transport
- Colloidal crystal
- Colloidal gold
- Eigencolloid
- Fullerenes
- Fungal-derived nanoparticles
- Gallium(II) selenide
- Icosahedral twins
- Indium selenide
- Liposome
- Magnetic immunoassay
- Magnetic nanoparticles
- Magnetic nanochains
- Micromeritics
- Nanobiotechnology
- Nanocrystalline silicon
- Nanofluid
- Nanogeoscience
- Nanomaterials
- Nanomedicine
- Nanoparticle deposition
- Nanoparticle Tracking Analysis
- Nanotechnology
- Patchy particles
- Photonic crystal
- Plasmon
- Platinum nanoparticles
- Quantum dot
- Self-assembly of nanoparticles
- Silicon
- Silver Nano
- Sol-gel
- Transparent material
- Upconverting nanoparticles
References
- U.S. Environmental Protection Agency (): "Module 3: Characteristics of Particles Particle Size Categories". From the EPA Website.
- Vert, M.; Doi, Y.; Hellwich, K. H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. O. (2012). "Terminology for biorelated polymers and applications (IUPAC Recommendations 2012)". Pure and Applied Chemistry. 84 (2): 377 410. doi:10.1351/PAC-REC-10-12-04.
- Vert, Michel; Doi, Yoshiharu; Hellwich, Karl-Heinz; Hess, Michael; Hodge, Philip; Kubisa, Przemyslaw; Rinaudo, Marguerite; Schué, François (11 January 2012). "Terminology for biorelated polymers and applications (IUPAC Recommendations 2012)". Pure and Applied Chemistry. 84 (2): 377–410. doi:10.1351/PAC-REC-10-12-04.
- Chae, Seung Yong; Park, Myun Kyu; Lee, Sang Kyung; Kim, Taek Young; Kim, Sang Kyu; Lee, Wan In (August 2003). "Preparation of Size-Controlled TiO 2 Nanoparticles and Derivation of Optically Transparent Photocatalytic Films". Chemistry of Materials. 15 (17): 3326–3331. doi:10.1021/cm030171d.
- Jacques Simonis, Jean; Koetzee Basson, Albertus (2011). "Evaluation of a low-cost ceramic micro-porous filter for elimination of common disease microorganisms". Physics and Chemistry of the Earth, Parts A/B/C. 36 (14–15): 1129–1134. doi:10.1016/j.pce.2011.07.064.
- Silvera Batista, C. A.; Larson, R. G.; Kotov, N. A. (9 October 2015). "Nonadditivity of nanoparticle interactions". Science. 350 (6257): 1242477–1242477. doi:10.1126/science.1242477. PMID 26450215.
- Cai, Wei; Nix, William D. (September 2016). Imperfections in Crystalline Solids. Cambridge Core. doi:10.1017/cbo9781316389508. ISBN 9781107123137. Retrieved 21 May 2020.
- Chen, Chien-Chun; Zhu, Chun; White, Edward R.; Chiu, Chin-Yi; Scott, M. C.; Regan, B. C.; Marks, Laurence D.; Huang, Yu; Miao, Jianwei (April 2013). "Three-dimensional imaging of dislocations in a nanoparticle at atomic resolution". Nature. 496 (7443): 74–77. Bibcode:2013Natur.496...74C. doi:10.1038/nature12009. PMID 23535594.
- Guo, Dan; Xie, Guoxin; Luo, Jianbin (8 January 2014). "Mechanical properties of nanoparticles: basics and applications". Journal of Physics D: Applied Physics. 47 (1): 013001. doi:10.1088/0022-3727/47/1/013001.
- Khan, Ibrahim; Saeed, Khalid; Khan, Idrees (November 2019). "Nanoparticles: Properties, applications and toxicities". Arabian Journal of Chemistry. 12 (7): 908–931. doi:10.1016/j.arabjc.2017.05.011.
- Carlton, C.E.; Rabenberg, L.; Ferreira, P.J. (September 2008). "On the nucleation of partial dislocations in nanoparticles". Philosophical Magazine Letters. 88 (9–10): 715–724. doi:10.1080/09500830802307641.
- Knauer, Andrea; Koehler, J. Michael (2016). "Explanation of the size dependent in-plane optical resonance of triangular silver nanoprisms". Physical Chemistry Chemical Physics. 18 (23): 15943–15949. doi:10.1039/c6cp00953k. PMID 27241479.
- MacNaught, Alan D.; Wilkinson, Andrew R., eds. (1997). Compendium of Chemical Terminology: IUPAC Recommendations (2nd ed.). Blackwell Science. ISBN 978-0865426849.
- Alemán, J. V.; Chadwick, A. V.; He, J.; Hess, M.; Horie, K.; Jones, R. G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; Penczek, S.; Stepto, R. F. T. (1 January 2007). "Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007)". Pure and Applied Chemistry. 79 (10): 1801–1829. doi:10.1351/pac200779101801.
- "ISO/TS 80004-2: Nanotechnologies Vocabulary Part 2: Nano-objects". International Organization for Standardization. 2015. Retrieved 18 January 2018.
- Fahlman, B. D. (2007). Materials Chemistry. Springer. pp. 282 283. ISBN 978-1-4020-6119-6.
- Pais, A. (2005). Subtle is the Lord: The Science and the Life of Albert Einstein. Oxford University Press. ISBN 978-0-19-280672-7. Retrieved 6 December 2016.
- Simakov, S. K. (2018). "Nano- and micron-sized diamond genesis in nature: An overview". Geoscience Frontiers. 9 (6): 1849–1858. doi:10.1016/j.gsf.2017.10.006.
- Simakov, S. K.; Kouchi, A.; Scribano, V.; Kimura, Y.; Hama, T.; Suzuki, N.; Saito, H.; Yoshizawa, T. (2015). "Nanodiamond Finding in the Hyblean Shallow Mantle Xenoliths". Nature: Scientific Reports. 5: 10765. doi:10.1038/srep10765.
- Plane, John M. C. (2012). "Cosmic dust in the earth's atmosphere". Chemical Society Reviews. 41: 6507–6518. doi:10.1039/C2CS35132C.
- Zook, Herbert A. (2001). "Spacecraft Measurements of the Cosmic Dust Flux". In Peucker-Ehrenbrink, B.; Schmitz, B. (eds.). Accretion of Extraterrestrial Matter Throughout Earth’s History. Boston, MA: Springer. doi:10.1007/978-1-4419-8694-8_5.
- "Nanotechnology Timeline | Nano". www.nano.gov. Retrieved 12 December 2016.
- Reiss, Gunter; Hutten, Andreas (2010). "Magnetic Nanoparticles". In Sattler, Klaus D. (ed.). Handbook of Nanophysics: Nanoparticles and Quantum Dots. CRC Press. pp. 2 1. ISBN 9781420075458.
- Khan, Firdos Alam (2012). Biotechnology Fundamentals. CRC Press. p. 328. ISBN 9781439820094.
- Faraday, Michael (1857). "Experimental relations of gold (and other metals) to light". Phil. Trans. R. Soc. Lond. 147: 145 181. Bibcode:1857RSPT..147..145F. doi:10.1098/rstl.1857.0011.
- Beilby, George Thomas (31 January 1904). "The effect of heat and of solvents on thin films of metal". Proceedings of the Royal Society of London. 72 (477–486): 226–235. Bibcode:1903RSPS...72..226B. doi:10.1098/rspl.1903.0046.
- Turner, T. (1908). "Transparent Silver and Other Metallic Films". Proceedings of the Royal Society A. 81 (548): 301–310. Bibcode:1908RSPSA..81..301T. doi:10.1098/rspa.1908.0084. JSTOR 93060.
- Granqvist, C.; Buhrman, R.; Wyns, J.; Sievers, A. (1976). "Far-Infrared Absorption in Ultrafine Al Particles". Physical Review Letters. 37 (10): 625 629. Bibcode:1976PhRvL..37..625G. doi:10.1103/PhysRevLett.37.625.
- Hayashi, C.; Uyeda, R & Tasaki, A. (1997). Ultra-fine particles: exploratory science and technology (1997 Translation of the Japan report of the related ERATO Project 1981 86). Noyes Publications.
- Kiss, L B; Söderlund, J; Niklasson, G A; Granqvist, C G (1 March 1999). "New approach to the origin of lognormal size distributions of nanoparticles". Nanotechnology. 10 (1): 25–28. Bibcode:1999Nanot..10...25K. doi:10.1088/0957-4484/10/1/006.
- Agam, M. A.; Guo, Q (2007). "Electron Beam Modification of Polymer Nanospheres". Journal of Nanoscience and Nanotechnology. 7 (10): 3615–9. doi:10.1166/jnn.2007.814. PMID 18330181.
- Kralj, Slavko; Makovec, Darko (27 October 2015). "Magnetic Assembly of Superparamagnetic Iron Oxide Nanoparticle Clusters into Nanochains and Nanobundles". ACS Nano. 9 (10): 9700–7. doi:10.1021/acsnano.5b02328. PMID 26394039.
- Choy J.H.; Jang E.S.; Won J.H.; Chung J.H.; Jang D.J. & Kim Y.W. (2004). "Hydrothermal route to ZnO nanocoral reefs and nanofibers". Appl. Phys. Lett. 84 (2): 287. Bibcode:2004ApPhL..84..287C. doi:10.1063/1.1639514.
- Sun, Y; Xia, Y (2002). "Shape-controlled synthesis of gold and silver nanoparticles". Science. 298 (5601): 2176–9. Bibcode:2002Sci...298.2176S. doi:10.1126/science.1077229. PMID 12481134.
- Murphy, C. J. (13 December 2002). "MATERIALS SCIENCE: Nanocubes and Nanoboxes". Science. 298 (5601): 2139–2141. doi:10.1126/science.1080007. PMID 12481122.
- Dufresne, Alain (June 2013). "Nanocellulose: a new ageless bionanomaterial". Materials Today. 16 (6): 220–227. doi:10.1016/j.mattod.2013.06.004.
- Le Corre, Déborah; Bras, Julien; Dufresne, Alain (10 May 2010). "Starch Nanoparticles: A Review". Biomacromolecules. 11 (5): 1139–1153. doi:10.1021/bm901428y. PMID 20405913.
- Luchini, Alessandra; Geho, David H.; Bishop, Barney; Tran, Duy; Xia, Cassandra; Dufour, Robert L.; Jones, Clinton D.; Espina, Virginia; Patanarut, Alexis; Zhou, Weidong; Ross, Mark M.; Tessitore, Alessandra; Petricoin, Emanuel F.; Liotta, Lance A. (January 2008). "Smart Hydrogel Particles: Biomarker Harvesting: One-Step Affinity Purification, Size Exclusion, and Protection against Degradation". Nano Letters. 8 (1): 350–361. doi:10.1021/nl072174l. PMC 2877922. PMID 18076201.
- Buzea, Cristina; Pacheco, Ivan I.; Robbie, Kevin (December 2007). "Nanomaterials and nanoparticles: Sources and toxicity". Biointerphases. 2 (4): MR17–MR71. arXiv:0801.3280. doi:10.1116/1.2815690. PMID 20419892.
- ASTM E 2456 06 Standard Terminology Relating to Nanotechnology
- Valenti G, Rampazzo R, Bonacchi S, Petrizza L, Marcaccio M, Montalti M, Prodi L, Paolucci F (2016). "Variable Doping Induces Mechanism Swapping in Electrogenerated Chemiluminescence of Ru(bpy)32+ Core Shell Silica Nanoparticles". J. Am. Chem. Soc. 138 (49): 15935–15942. doi:10.1021/jacs.6b08239. PMID 27960352.
- Gubin, Sergey P. (2009). Magnetic nanoparticles. Wiley-VCH. ISBN 978-3-527-40790-3.
- Vollath, Dieter; Fischer, Franz Dieter; Holec, David (23 August 2018). "Surface energy of nanoparticles – influence of particle size and structure". Beilstein Journal of Nanotechnology. 9: 2265–2276. doi:10.3762/bjnano.9.211. PMC 6122122. PMID 30202695.
- Jiang, Q.; Liang, L. H.; Zhao, D. S. (July 2001). "Lattice Contraction and Surface Stress of fcc Nanocrystals". The Journal of Physical Chemistry B. 105 (27): 6275–6277. doi:10.1021/jp010995n.
- Courtney, Thomas H. (2000). Mechanical behavior of materials (2nd ed.). Boston: McGraw Hill. ISBN 0-07-028594-2. OCLC 41932585.
- Ramos, Manuel; Ortiz-Jordan, Luis; Hurtado-Macias, Abel; Flores, Sergio; Elizalde-Galindo, José T.; Rocha, Carmen; Torres, Brenda; Zarei-Chaleshtori, Maryam; Chianelli, Russell R. (January 2013). "Hardness and Elastic Modulus on Six-Fold Symmetry Gold Nanoparticles". Materials. 6 (1): 198–205. doi:10.3390/ma6010198. PMC 5452105. PMID 28809302.
- Oh, Sang Ho; Legros, Marc; Kiener, Daniel; Dehm, Gerhard (February 2009). "In situ observation of dislocation nucleation and escape in a submicrometre aluminium single crystal". Nature Materials. 8 (2): 95–100. doi:10.1038/nmat2370. PMID 19151703.
- Feruz, Yosi; Mordehai, Dan (January 2016). "Towards a universal size-dependent strength of face-centered cubic nanoparticles". Acta Materialia. 103: 433–441. doi:10.1016/j.actamat.2015.10.027.
- Kulik, Andrzej; Kis, Andras; Gremaud, Gérard; Hengsberger, Stefan; Luengo, Gustavo; Zysset, Philippe; Forró, László (2007), Bhushan, Bharat (ed.), "Nanoscale Mechanical Properties – Measuring Techniques and Applications", Springer Handbook of Nanotechnology, Springer Handbooks, Springer, pp. 1107–1136, doi:10.1007/978-3-540-29857-1_36, ISBN 978-3-540-29857-1
- Buffat, Ph.; Borel, J.-P. (1976). "Size effect on the melting temperature of gold particles". Physical Review A. 13 (6): 2287–2298. Bibcode:1976PhRvA..13.2287B. doi:10.1103/PhysRevA.13.2287.
- Hewakuruppu, Y. L.; Dombrovsky, L. A.; Chen, C.; Timchenko, V.; Jiang, X.; Baek, S.; Taylor, R. A. (2013). "Plasmonic "pump probe" method to study semi-transparent nanofluids". Applied Optics. 52 (24): 6041–50. Bibcode:2013ApOpt..52.6041H. doi:10.1364/AO.52.006041. PMID 24085009.
- Wu, Jiang; Yu, Peng; Susha, Andrei S.; Sablon, Kimberly A.; Chen, Haiyuan; Zhou, Zhihua; Li, Handong; Ji, Haining; Niu, Xiaobin (1 April 2015). "Broadband efficiency enhancement in quantum dot solar cells coupled with multispiked plasmonic nanostars". Nano Energy. 13: 827–835. doi:10.1016/j.nanoen.2015.02.012.
- Taylor, Robert A; Otanicar, Todd; Rosengarten, Gary (2012). "Nanofluid-based optical filter optimization for PV/T systems". Light: Science & Applications. 1 (10): e34. Bibcode:2012LSA.....1E..34T. doi:10.1038/lsa.2012.34.
- Taylor, Robert A.; Otanicar, Todd P.; Herukerrupu, Yasitha; Bremond, Fabienne; Rosengarten, Gary; Hawkes, Evatt R.; Jiang, Xuchuan; Coulombe, Sylvain (2013). "Feasibility of nanofluid-based optical filters". Applied Optics. 52 (7): 1413–22. Bibcode:2013ApOpt..52.1413T. doi:10.1364/AO.52.001413. PMID 23458793.
- Taylor, Robert A; Phelan, Patrick E; Otanicar, Todd P; Adrian, Ronald; Prasher, Ravi (2011). "Nanofluid optical property characterization: Towards efficient direct absorption solar collectors". Nanoscale Research Letters. 6 (1): 225. Bibcode:2011NRL.....6..225T. doi:10.1186/1556-276X-6-225. PMC 3211283. PMID 21711750.
- Valenti G, Rampazzo E, Kesarkar S, Genovese D, Fiorani A, Zanut A, Palomba F, Marcaccio M, Paolucci F, Prodi L (2018). "Electrogenerated chemiluminescence from metal complexes-based nanoparticles for highly sensitive sensors applications". Coordination Chemistry Reviews. 367: 65–81. doi:10.1016/j.ccr.2018.04.011.
- Taylor, Robert; Coulombe, Sylvain; Otanicar, Todd; Phelan, Patrick; Gunawan, Andrey; Lv, Wei; Rosengarten, Gary; Prasher, Ravi; Tyagi, Himanshu (2013). "Small particles, big impacts: A review of the diverse applications of nanofluids". Journal of Applied Physics. 113 (1): 011301–011301–19. Bibcode:2013JAP...113a1301T. doi:10.1063/1.4754271.
- Ghosh Chaudhuri, Rajib; Paria, Santanu (11 April 2012). "Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications". Chemical Reviews. 112 (4): 2373–2433. doi:10.1021/cr100449n. PMID 22204603.
- Loo, Jacky Fong-Chuen; Chien, Yi-Hsin; Yin, Feng; Kong, Siu-Kai; Ho, Ho-Pui; Yong, Ken-Tye (December 2019). "Upconversion and downconversion nanoparticles for biophotonics and nanomedicine". Coordination Chemistry Reviews. 400: 213042. doi:10.1016/j.ccr.2019.213042.
- Yu, Peng; Yao, Yisen; Wu, Jiang; Niu, Xiaobin; Rogach, Andrey L.; Wang, Zhiming (December 2017). "Effects of Plasmonic Metal Core -Dielectric Shell Nanoparticles on the Broadband Light Absorption Enhancement in Thin Film Solar Cells". Scientific Reports. 7 (1): 7696. Bibcode:2017NatSR...7.7696Y. doi:10.1038/s41598-017-08077-9. PMC 5550503. PMID 28794487.
- Whitesides, G.M.; et al. (1991). "Molecular Self-Assembly and Nanochemistry: A Chemical Strategy for the Synthesis of Nanostructures". Science. 254 (5036): 1312–1319. Bibcode:1991Sci...254.1312W. doi:10.1126/science.1962191. PMID 1962191.
- Dabbs D. M, Aksay I.A.; Aksay (2000). "Self-Assembled Ceramics". Annu. Rev. Phys. Chem. 51: 601–22. Bibcode:2000ARPC...51..601D. doi:10.1146/annurev.physchem.51.1.601. PMID 11031294.
- Anandkumar, Mariappan; Bhattacharya, Saswata; Deshpande, Atul Suresh (2019). "Low temperature synthesis and characterization of single phase multi-component fluorite oxide nanoparticle sols". RSC Advances. 9 (46): 26825–26830. doi:10.1039/C9RA04636D.
- Saito, Tsuguyuki; Kimura, Satoshi; Nishiyama, Yoshiharu; Isogai, Akira (August 2007). "Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose". Biomacromolecules. 8 (8): 2485–2491. doi:10.1021/bm0703970. PMID 17630692.
- Fan, Yimin; Saito, Tsuguyuki; Isogai, Akira (17 March 2010). "Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization". Carbohydrate Polymers. 79 (4): 1046–1051. doi:10.1016/j.carbpol.2009.10.044.
- Habibi, Youssef (2014). "Key advances in the chemical modification of nanocelluloses". Chem. Soc. Rev. 43 (5): 1519–1542. doi:10.1039/c3cs60204d. PMID 24316693.
- Granqvist, C. G.; Buhrman, R. A. (1976). "Ultrafine metal particles". Journal of Applied Physics. 47 (5): 2200 2219. Bibcode:1976JAP....47.2200G. doi:10.1063/1.322870.
- Hahn, H.; Averback, R. S. (1990). "The production of nanocrystalline powders by magnetron sputtering". Journal of Applied Physics. 67 (2): 1113 1115. Bibcode:1990JAP....67.1113H. doi:10.1063/1.345798.
- Wang, Jian-Ping; Bai, Jianmin (2005). "High-magnetic-moment core-shell-type FeCo Au AgFeCo Au Ag nanoparticles". Appl. Phys. Lett. 87: 152502. doi:10.1063/1.2089171.
- Hennes, M.; Lotnyk, A.; Mayr, S. G. (2014). "Plasma-assisted synthesis and high-resolution characterization of anisotropic elemental and bimetallic core shell magnetic nanoparticles". Beilstein J. Nanotechnol. 5: 466–475. doi:10.3762/bjnano.5.54. PMC 3999878. PMID 24778973.
- Llamosa, D.; Ruano, M.; Martínez, L.; Mayoral, A.; Roman, E.; García-Hernández, M.; Huttel, Y. (2014). "The ultimate step towards a tailored engineering of core@shell and core@shell@shell nanoparticles". Nanoscale. 6 (22): 13483–13486. Bibcode:2014Nanos...613483L. doi:10.1039/c4nr02913e. PMID 25180699.
- Michelakaki, Irini; Boukos, Nikos; Dragatogiannis, Dimitrios A; Stathopoulos, Spyros; Charitidis, Costas A; Tsoukalas, Dimitris (27 June 2018). "Synthesis of hafnium nanoparticles and hafnium nanoparticle films by gas condensation and energetic deposition". Beilstein Journal of Nanotechnology. 9: 1868–1880. doi:10.3762/bjnano.9.179. PMC 6036986. PMID 30013881.
- Belloni, J.; Mostafavi, M.; Remita, H.; Marignier, J. L.; Delcourt, A. M. O. (1998). "Radiation-induced synthesis of mono- and multi-metallic clusters and nanocolloids". New Journal of Chemistry. 22 (11): 1239 1255. doi:10.1039/A801445K.
- Brinker, C.J. & Scherer, G.W. (1990). Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. Academic Press. ISBN 978-0-12-134970-7.
- Hench, L. L.; West, J. K. (1990). "The sol-gel process". Chemical Reviews. 90: 33–72. doi:10.1021/cr00099a003.
- Klein, L. (1994). Sol-Gel Optics: Processing and Applications. Springer Verlag. ISBN 978-0-7923-9424-2. Retrieved 6 December 2016.
- Corriu, Robert & Anh, Nguyên Trong (2009). Molecular Chemistry of Sol-Gel Derived Nanomaterials. John Wiley and Sons. ISBN 978-0-470-72117-9.
- Sadri, R. (15 October 2017). "Study of environmentally friendly and facile functionalization of graphene nanoplatelet and its application in convective heat transfer". Energy Conversion and Management. 150: 26–36. doi:10.1016/j.enconman.2017.07.036.
- Prime, KL; Whitesides, GM (1991). "Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces". Science. 252 (5009): 1164–7. Bibcode:1991Sci...252.1164P. doi:10.1126/science.252.5009.1164. PMID 2031186.
- Liu, Wenhao; Greytak, Andrew B.; Lee, Jungmin; Wong, Cliff R.; Park, Jongnam; Marshall, Lisa F.; Jiang, Wen; Curtin, Peter N.; Ting, Alice Y.; Nocera, Daniel G.; Fukumura, Dai; Jain, Rakesh K.; Bawendi, Moungi G. (20 January 2010). "Compact Biocompatible Quantum Dots via RAFT-Mediated Synthesis of Imidazole-Based Random Copolymer Ligand". Journal of the American Chemical Society. 132 (2): 472–483. doi:10.1021/ja908137d. PMC 2871316. PMID 20025223.
- Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E (2002). "Nanocrystal targeting in vivo". Proceedings of the National Academy of Sciences of the United States of America. 99 (20): 12617–12621. Bibcode:2002PNAS...9912617A. doi:10.1073/pnas.152463399. PMC 130509. PMID 12235356.
- Hoshino, A; Fujioka, K; Oku, T; Nakamura, S; Suga, M; Yamaguchi, Y; Suzuki, K; Yasuhara, M; Yamamoto, K (2004). "Quantum dots targeted to the assigned organelle in living cells". Microbiology and Immunology. 48 (12): 985–94. doi:10.1111/j.1348-0421.2004.tb03621.x. PMID 15611617.
- Suzuki, KG; Fujiwara, TK; Edidin, M; Kusumi, A (2007). "Dynamic recruitment of phospholipase C at transiently immobilized GPI-anchored receptor clusters induces IP3 Ca2+ signaling: single-molecule tracking study 2". The Journal of Cell Biology. 177 (4): 731–42. doi:10.1083/jcb.200609175. PMC 2064217. PMID 17517965.
- Sung, KM; Mosley, DW; Peelle, BR; Zhang, S; Jacobson, JM (2004). "Synthesis of monofunctionalized gold nanoparticles by fmoc solid-phase reactions". Journal of the American Chemical Society. 126 (16): 5064–5. doi:10.1021/ja049578p. PMID 15099078.
- Fu, A; Micheel, CM; Cha, J; Chang, H; Yang, H; Alivisatos, AP (2004). "Discrete nanostructures of quantum dots/Au with DNA". Journal of the American Chemical Society. 126 (35): 10832–3. doi:10.1021/ja046747x. PMID 15339154.
- Howarth, M; Liu, W; Puthenveetil, S; Zheng, Y; Marshall, LF; Schmidt, MM; Wittrup, KD; Bawendi, MG; Ting, AY (2008). "Monovalent, reduced-size quantum dots for imaging receptors on living cells". Nature Methods. 5 (5): 397–9. doi:10.1038/nmeth.1206. PMC 2637151. PMID 18425138.
- "Nanoparticles play at being red blood cells". Archived from the original on 1 July 2011. Retrieved 1 July 2011.
- Onoda, G.Y. Jr.; Hench, L.L., eds. (1979). Ceramic Processing Before Firing. New York: Wiley & Sons. ISBN 978-0-471-65410-0.
- Aksay, I.A.; Lange, F.F.; Davis, B.I. (1983). "Uniformity of Al2O3-ZrO2 Composites by Colloidal Filtration". J. Am. Ceram. Soc. 66 (10): C 190. doi:10.1111/j.1151-2916.1983.tb10550.x.
- Franks, G.V. & Lange, F.F. (1996). "Plastic-to-Brittle Transition of Saturated, Alumina Powder Compacts". J. Am. Ceram. Soc. 79 (12): 3161 3168. doi:10.1111/j.1151-2916.1996.tb08091.x.
- Evans, A.G. & Davidge, R.W. (1969). "The strength and fracture of fully dense polycrystalline magnesium oxide". Phil. Mag. 20 (164): 373 388. Bibcode:1969PMag...20..373E. doi:10.1080/14786436908228708.
- Evans, A. G.; Davidge, R. W. (1970). "The strength and oxidation of reaction-sintered silicon nitride". J. Mater. Sci. 5 (4): 314 325. Bibcode:1970JMatS...5..314E. doi:10.1007/BF02397783.
- Lange, F. F.; Metcalf, M. (June 1983). "Processing-Related Fracture Origins: II, Agglomerate Motion and Cracklike Internal Surfaces Caused by Differential Sintering". Journal of the American Ceramic Society. 66 (6): 398–406. doi:10.1111/j.1151-2916.1983.tb10069.x.
- Evans, A.G. (1987). "Considerations of Inhomogeneity Effects in Sintering". J. Am. Ceram. Soc. 65 (10): 497–501. doi:10.1111/j.1151-2916.1982.tb10340.x.
- Hassellöv, Martin; Readman, James W.; Ranville, James F.; Tiede, Karen (July 2008). "Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles". Ecotoxicology. 17 (5): 344–361. doi:10.1007/s10646-008-0225-x. PMID 18483764.
- Powers, Kevin W.; Palazuelos, Maria; Moudgil, Brij M.; Roberts, Stephen M. (January 2007). "Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies". Nanotoxicology. 1 (1): 42–51. doi:10.1080/17435390701314902.
- Tiede, Karen; Boxall, Alistair B.A.; Tear, Steven P.; Lewis, John; David, Helen; Hassellöv, Martin (July 2008). "Detection and characterization of engineered nanoparticles in food and the environment" (PDF). Food Additives & Contaminants: Part A. 25 (7): 795–821. doi:10.1080/02652030802007553. PMID 18569000.
- Linsinger, Thomas P.J.; Roebben, Gert; Solans, Conxita; Ramsch, Roland (January 2011). "Reference materials for measuring the size of nanoparticles". TrAC Trends in Analytical Chemistry. 30 (1): 18–27. doi:10.1016/j.trac.2010.09.005.
- Zoroddu, Maria Antonietta; Medici, Serenella; Ledda, Alessia; Nurchi, Valeria Marina; Peana, Joanna I. Lachowicz and Massimiliano; Peana, M (31 October 2014). "Toxicity of Nanoparticles". Current Medicinal Chemistry. doi:10.2174/0929867321666140601162314. PMID 25306903.
- Crisponi, G.; Nurchi, V.M.; Lachowicz, J.; Peana, M.; Medici, S.; Zoroddu, M.A. (2017). Chapter 18 - Toxicity of Nanoparticles: Etiology and Mechanisms, in Antimicrobial Nanoarchitectonics. ELSEVIER. pp. 511 546. doi:10.1016/B978-0-323-52733-0.00018-5. ISBN 9780323527330.
- Mnyusiwalla, Anisa; Daar, Abdallah S; Singer, Peter A (1 March 2003). "'Mind the gap': science and ethics in nanotechnology" (PDF). Nanotechnology. 14 (3): R9–R13. doi:10.1088/0957-4484/14/3/201.
- "Toxic Nanoparticles Might be Entering Human Food Supply, MU Study Finds". University of Missouri. 22 August 2013. Retrieved 23 August 2013.
- Ying, Jackie (2001). Nanostructured Materials. New York: Academic Press. ISBN 978-0-12-744451-2. Retrieved 6 December 2016.
- Nanotechnologies: 6. What are potential harmful effects of nanoparticles? europa.eu
- Thake, T.H.F; Webb, J.R; Nash, A.; Rappoport, J.Z.; Notman, R. (2013). "Permeation of polystyrene nanoparticles across model lipid bilayer membranes". Soft Matter. 9 (43): 10265 10274. Bibcode:2013SMat....910265T. doi:10.1039/c3sm51225h.
- Greulich, C.; Diendorf, J.; Simon, T.; Eggeler, G.; Epple, M.; Köller, M. (January 2011). "Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells". Acta Biomaterialia. 7 (1): 347–354. doi:10.1016/j.actbio.2010.08.003. PMID 20709196.
- Hanley, Cory; Thurber, Aaron; Hanna, Charles; Punnoose, Alex; Zhang, Jianhui; Wingett, Denise G. (December 2009). "The Influences of Cell Type and ZnO Nanoparticle Size on Immune Cell Cytotoxicity and Cytokine Induction". Nanoscale Research Letters. 4 (12): 1409–1420. Bibcode:2009NRL.....4.1409H. doi:10.1007/s11671-009-9413-8. PMID 20652105.
- Vines T, Faunce T (2009). "Assessing the safety and cost-effectiveness of early nanodrugs". Journal of Law and Medicine. 16 (5): 822–45. PMID 19554862.
- Benson, Heather AE; Sarveiya, Vikram; Risk, Stacey; Roberts, Michael S (2005). "Influence of anatomical site and topical formulation on skin penetration of sunscreens". Therapeutics and Clinical Risk Management. 1 (3): 209–218. PMC 1661631. PMID 18360561.
- Howard, V. (2009). "Statement of Evidence: Particulate Emissions and Health (An Bord Plenala, on Proposed Ringaskiddy Waste-to-Energy Facility)." Retrieved 26 April 2011.
- Pieters, N (March 2015). "Blood Pressure and Same-Day Exposure to Air Pollution at School: Associations with Nano-Sized to Coarse PM in Children". Environmental Health Perspectives. 123 (7): 737–742. doi:10.1289/ehp.1408121. PMC 4492263. PMID 25756964.
- Mapanao, Ana Katrina; Giannone, Giulia; Summa, Maria; Ermini, Maria Laura; Zamborlin, Agata; Santi, Melissa; Cassano, Domenico; Bertorelli, Rosalia; Voliani, Valerio (2020). "Biokinetics and clearance of inhaled gold ultrasmall-in-nano architectures". Nanoscale Advances. doi:10.1039/D0NA00521E.
- Cassano, Domenico; Mapanao, Ana-Katrina; Summa, Maria; Vlamidis, Ylea; Giannone, Giulia; Santi, Melissa; Guzzolino, Elena; Pitto, Letizia; Poliseno, Laura; Bertorelli, Rosalia; Voliani, Valerio (21 October 2019). "Biosafety and Biokinetics of Noble Metals: The Impact of Their Chemical Nature". ACS Applied Bio Materials. 2 (10): 4464–4470. doi:10.1021/acsabm.9b00630.
- "Nanomaterials EPA is Assessing". Environmental Protection Agency. Retrieved 6 February 2013.

- Susan Wayland and Penelope Fenner-Crisp. Reducing Pesticide Risks: A Half Century of Progress. EPA Alumni Association. March 2016.
- "The Textiles Nanotechnology Laboratory". nanotextiles.human.cornell.edu. Retrieved 6 December 2016.
- Evans, B. (January 2018). "Nano-particle drag prediction at low Reynolds number using a direct Boltzmann–BGK solution approach" (PDF). Journal of Computational Physics. 352: 123–141. Bibcode:2018JCoPh.352..123E. doi:10.1016/j.jcp.2017.09.038.
- Hafezi, F.; Ransing, R. S.; Lewis, R. W. (14 September 2017). "The calculation of drag on nano-cylinders: The calculation of drag on nano-cylinders" (PDF). International Journal for Numerical Methods in Engineering. 111 (11): 1025–1046. Bibcode:2017IJNME.111.1025H. doi:10.1002/nme.5489.
- Salata, OV (2004). "Applications of nanoparticles in biology and medicine". Journal of Nanobiotechnology. 2 (1): 3. doi:10.1186/1477-3155-2-3. PMC 419715. PMID 15119954.
- Hubler, A.; Osuagwu, O. (2010). "Digital quantum batteries: Energy and information storage in nanovacuum tube arrays". Complexity: NA. doi:10.1002/cplx.20306.
- Stephenson, C.; Hubler, A. (2015). "Stability and conductivity of self assembled wires in a transverse electric field". Sci. Rep. 5: 15044. Bibcode:2015NatSR...515044S. doi:10.1038/srep15044. PMC 4604515. PMID 26463476.
- Hubler, A.; Lyon, D. (2013). "Gap size dependence of the dielectric strength in nano vacuum gaps". IEEE Transactions on Dielectrics and Electrical Insulation. 20 (4): 1467 1471. doi:10.1109/TDEI.2013.6571470.
- Omidvar, A. (2016). "Metal-enhanced fluorescence of graphene oxide by palladium nanoparticles in the blue-green part of the spectrum". Chinese Physics B. 25 (11): 118102. Bibcode:2016ChPhB..25k8102O. doi:10.1088/1674-1056/25/11/118102.
- Rashidian V, M.R. (2017). "Investigating the extrinsic size effect of palladium and gold spherical nanoparticles". Optical Materials. 64: 413–420. Bibcode:2017OptMa..64..413R. doi:10.1016/j.optmat.2017.01.014.
- Omidvar, A. (2018). "Enhancing the nonlinear optical properties of graphene oxide by repairing with palladium nanoparticles". Physica E: Low-dimensional Systems and Nanostructures. 103: 239–245. Bibcode:2018PhyE..103..239O. doi:10.1016/j.physe.2018.06.013.
- Duarte, F. J.; James, R. O. (2003). "Tunable solid-state lasers incorporating dye-doped polymer-nanoparticle gain media". Opt. Lett. 28 (21): 2088–90. Bibcode:2003OptL...28.2088D. doi:10.1364/OL.28.002088. PMID 14587824.
- Singh, BN; Prateeksha, Gupta VK; Chen, J; Atanasov, AG (2017). "Organic Nanoparticle-Based Combinatory Approaches for Gene Therapy". Trends Biotechnol. 35 (12): 1121–1124. doi:10.1016/j.tibtech.2017.07.010. PMID 28818304..
- Wang, Zhenming; Wang, Zhefeng; Lu, William Weijia; Zhen, Wanxin; Yang, Dazhi; Peng, Songlin (October 2017). "Novel biomaterial strategies for controlled growth factor delivery for biomedical applications". NPG Asia Materials. 9 (10): e435–e435. doi:10.1038/am.2017.171.
- Jóźwik, Artur; Marchewka, Joanna; Strzałkowska, Nina; Horbańczuk, Jarosław; Szumacher-Strabel, Małgorzata; Cieślak, Adam; Lipińska-Palka, Paulina; Józefiak, Damian; Kamińska, Agnieszka; Atanasov, Atanas (11 May 2018). "The Effect of Different Levels of Cu, Zn and Mn Nanoparticles in Hen Turkey Diet on the Activity of Aminopeptidases". Molecules. 23 (5): 1150. doi:10.3390/molecules23051150. PMID 29751626.
- Cheraghian, Goshtasp; Wistuba, Michael P. (8 July 2020). "Ultraviolet aging study on bitumen modified by a composite of clay and fumed silica nanoparticles". Scientific Reports. 10 (1): 1–17. doi:10.1038/s41598-020-68007-0.
- "Sunscreen". U.S. Food and Drug Administration. Retrieved 6 December 2016.
- Mitchnick, Mark A.; Fairhurst, David; Pinnell, Sheldon R. (January 1999). "Microfine zinc oxide (Z-Cote) as a photostable UVA/UVB sunblock agent". Journal of the American Academy of Dermatology. 40 (1): 85–90. doi:10.1016/s0190-9622(99)70532-3. PMID 9922017.
- Heim, J; Felder, E; Tahir, MN; Kaltbeitzel, A; Heinrich, UR; Brochhausen, C; Mailänder, V; Tremel, W; Brieger, J (21 May 2015). "Genotoxic effects of zinc oxide nanoparticles". Nanoscale. 7 (19): 8931–8. Bibcode:2015Nanos...7.8931H. doi:10.1039/c5nr01167a. PMID 25916659.
- Wang, Bing; Zhang, Yuying; Mao, Zhengwei; Yu, Dahai; Gao, Changyou (1 August 2014). "Toxicity of ZnO Nanoparticles to Macrophages Due to Cell Uptake and Intracellular Release of Zinc Ions". Journal of Nanoscience and Nanotechnology. 14 (8): 5688–5696. doi:10.1166/jnn.2014.8876. PMID 25935990.
- Gosens, I; Kermanizadeh, A; Jacobsen, NR; Lenz, AG; Bokkers, B; de Jong, WH; Krystek, P; Tran, L; Stone, V; Wallin, H; Stoeger, T; Cassee, FR (2015). "Comparative hazard identification by a single dose lung exposure of zinc oxide and silver nanomaterials in mice". PLOS ONE. 10 (5): e0126934. Bibcode:2015PLoSO..1026934G. doi:10.1371/journal.pone.0126934. PMC 4429007. PMID 25966284.
- Hanagata, N; Morita, H (2015). "Calcium ions rescue human lung epithelial cells from the toxicity of zinc oxide nanoparticles". The Journal of Toxicological Sciences. 40 (5): 625–35. doi:10.2131/jts.40.625. PMID 26354379.
- Kim, Young Hee; Kwak, Kyung A; Kim, Tae Sung; Seok, Ji Hyeon; Roh, Hang Sik; Lee, Jong-Kwon; Jeong, Jayoung; Meang, Eun Ho; Hong, Jeong-sup; Lee, Yun Seok; Kang, Jin Seok (30 June 2015). "Retinopathy Induced by Zinc Oxide Nanoparticles in Rats Assessed by Micro-computed Tomography and Histopathology". Toxicological Research. 31 (2): 157–163. doi:10.5487/TR.2015.31.2.157. PMC 4505346. PMID 26191382.
- Moridian, M.; Khorsandi, L.; Talebi, A. R. (2015). "Morphometric and stereological assessment of the effects of zinc oxide nanoparticles on the mouse testicular tissue". Bratislava Medical Journal. 116 (05): 321–325. doi:10.4149/bll_2015_060. PMID 25924642.
Further reading
- Jackie Y. Ying (2001). Nanostructured Materials. Academic Press. pp. 5–. ISBN 978-0-12-744451-2.
- Nanoparticles Used in Solar Energy Conversion (ScienceDaily).
- "Nanoparticles: An occupational hygiene review" by RJ Aitken and others. Health and Safety Executive Research Report 274/2004
- "EMERGNANO: A review of completed and near completed environment, health and safety research on nanomaterials and nanotechnology" by RJ Aitken and others.
- High transmission Tandem DMA for nanoparticle studies by SEADM, 2014.
External links
| Wikibooks has a book on the topic of: Nanotechnology |
- Nanohedron.com images of nanoparticles
- Lectures on All Phases of Nanoparticle Science and Technology
- ENPRA – Risk Assessment of Engineered NanoParticles EC FP7 Project led by the Institute of Occupational Medicine