Kojic acid
Kojic acid is a chelation agent produced by several species of fungi, especially Aspergillus oryzae, which has the Japanese common name koji.[2][3][4] Kojic acid is a by-product in the fermentation process of malting rice, for use in the manufacturing of sake, the Japanese rice wine.[2] It is a mild inhibitor of the formation of pigment in plant and animal tissues, and is used in food and cosmetics to preserve or change colors of substances.[5] It forms a bright red complex with ferric ions.
 | |
| Names | |
|---|---|
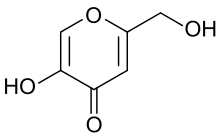
| IUPAC name
5-Hydroxy-2-(hydroxymethyl)-4H-pyran-4-one | |
| Other names
Kojic acid, 5-Hydroxy-2-(hydroxymethyl)-4-pyrone, 2-Hydroxymethyl-5-hydroxy-γ-pyrone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.203 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H6O4 | |
| Molar mass | 142.11 g/mol |
| Appearance | white |
| Melting point | 152 to 155 °C (306 to 311 °F; 425 to 428 K) |
| Slight | |
| Acidity (pKa) | 9.40[1] |
| Hazards | |
| R-phrases (outdated) | R36/37/38 |
| S-phrases (outdated) | S22, S24/25 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis
13C-Labeling studies have revealed at least two pathways to kojic acid. In the usual route, dehydratase enzymes convert glucose to kojic acid. Pentoses are also viable precursors in which case dihydroxyacetone is invoked as an intermediate.[2]
Applications
Kojic acid may be used on cut fruits to prevent oxidative browning, in seafood to preserve pink and red colors, and in cosmetics to lighten skin. As an example of the latter, it is used to treat skin diseases like melasma.[6] Kojic acid also has antibacterial and antifungal properties. The cocrystals of kojic acid with quercetin were found to have two times better cytotoxic activity to human cervical cancer cells (HeLa) and human colon cancer cells (Caco-2) in comparison with quercetin itself.[7]
Other effects
Kojic acid has been shown to protect Chinese hamster ovary cells against ionizing radiation-induced damage. Dogs pretreated with kojic acid after whole-body exposure to a lethal dose of 3 Gy gamma radiation had a 51-day survival rate of 66.7% versus the dogs in the 3 Gy irradiation only group, which all died within 16 days of postirradiation.[8]
References
- Bjerrum, J., et al. Stability Constants, Chemical Society, London, 1958.
- Bentley, R. (2006). "From miso, sake and shoyu to cosmetics: a century of science for kojic acid". Nat. Prod. Rep. 23: 1046–1062. doi:10.1039/b603758p.
- Yabuta T (1924). "The constitution of kojic acid, a gamma-pyrone derivative formed by Aspergillus oryzae from carbohydrates". Journal of the Chemical Society. 125: 575–587. doi:10.1039/ct9242500575.
- Parvez, Shoukat; Kang, Moonkyu; Chung, Hwan-Suck; Cho, Chongwoon; Hong, Moo-Chang; Shin, Min-Kyu; Bae, Hyunsu (2006). "Survey and mechanism of skin depigmenting and lightening agents". Phytotherapy Research. 20: 921-934. doi:10.1002/ptr.1954.CS1 maint: multiple names: authors list (link)
- Kojic acid and enzymatic browning Archived 2008-03-04 at the Wayback Machine
- Melasma Archived 2009-12-23 at the Wayback Machine, American Academy of Dermatology
- Veverka, M., Dubaj, T., Gallovič, J., Jorík, V., Veverková, E., Danihelová, M., & Šimon, P. (2015). Cocrystals of quercetin: synthesis, characterization, and screening of biological activity. Monatshefte für Chemie-Chemical Monthly,146(1), 99-109 doi:10.1007/s00706-014-1314-6
- Wang, Kai; Li, Peng-Fei; Han, Chun-Guang; Du, Li; Liu, Chao; Hu, Ming; Lian, Shi-Jie; Liu, Yong-Xue (2014). "Protective Effects of Kojic Acid on the Periphery Blood and Survival of Beagle Dogs after Exposure to a Lethal Dose of Gamma Radiation". Radiation Research. 182 (6): 666–673. doi:10.1667/RR13823.1.