Mosasaur
Mosasaurs (from Latin Mosa meaning the 'Meuse river', and Greek σαύρος sauros meaning 'lizard') comprise a group of extinct, large marine reptiles containing 38 genera in total. Their first fossil remains were discovered in a limestone quarry at Maastricht on the Meuse in 1764. Mosasaurs probably evolved from an extinct group of aquatic lizards[1] known as aigialosaurs in the Earliest Late Cretaceous. During the last 20 million years of the Cretaceous period (Turonian-Maastrichtian ages), with the extinction of the ichthyosaurs and pliosaurs, mosasaurs became the dominant marine predators. They became extinct as a result of the K-Pg event at the end of the Cretaceous period, about 66 million years ago.
| Mosasaurs | |
|---|---|
 | |
| Mounted skeleton of a plioplatecarpine (Plesioplatecarpus planifrons), Rocky Mountain Dinosaur Resource Center | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Clade: | Pythonomorpha |
| Superfamily: | †Mosasauroidea Gervais, 1853 |
| Subgroups | |
| |
Description

Mosasaurs breathed air, were powerful swimmers, and were well-adapted to living in the warm, shallow inland seas prevalent during the Late Cretaceous period. Mosasaurs were so well adapted to this environment that they most likely gave birth to live young, rather than returning to the shore to lay eggs as sea turtles do.[2]
The smallest-known mosasaur was Dallasaurus turneri, which was less than 1 m (3.3 ft) long. Larger mosasaurs were more typical, with many species growing longer than 4 m (13 ft). Mosasaurus hoffmannii, the largest known species, may have reached up to 17 m (56 ft) in length.[3] Currently, the largest publicly exhibited mosasaur skeleton in the world is on display at the Canadian Fossil Discovery Centre in Morden, Manitoba. The specimen, nicknamed "Bruce", is just over 13 m (43 ft) long.[4]
Mosasaurs had a body shape similar to that of modern-day monitor lizards (varanids), but were more elongated and streamlined for swimming. Their limb bones were reduced in length and their paddles were formed by webbing between their long finger and toe bones. Their tails were broad, and supplied their locomotive power. Until recently, mosasaurs were assumed to have swum in a method similar to the one used today by conger eels and sea snakes, undulating their entire bodies from side to side. However, new evidence suggests that many advanced mosasaurs had large, crescent-shaped flukes on the ends of their tails, similar to those of sharks and some ichthyosaurs. Rather than use snake-like undulations, their bodies probably remained stiff to reduce drag through the water, while their tails provided strong propulsion.[5] These animals may have lurked and pounced rapidly and powerfully on passing prey, rather than chasing after it.[6]
Early reconstructions showed mosasaurs with dorsal crests running the length of their bodies, which were based on misidentified remains of tracheal cartilage. By the time this error was discovered, depicting mosasaurs with such crests in artwork had already become a trend.[7][8]
Paleobiology


Mosasaurs had double-hinged jaws and flexible skulls (much like those of snakes), which enabled them to gulp down their prey almost whole. A skeleton of Tylosaurus proriger from South Dakota included remains of the diving seabird Hesperornis, a marine bony fish, a possible shark, and another, smaller mosasaur (Clidastes). Mosasaur bones have also been found with shark teeth embedded in them.
One of the food items of mosasaurs were ammonites, molluscs with shells similar to those of Nautilus, which were abundant in the Cretaceous seas. Holes have been found in fossil shells of some ammonites, mainly Pachydiscus and Placenticeras. These were once interpreted as a result of limpets attaching themselves to the ammonites, but the triangular shape of the holes, their size, and their presence on both sides of the shells, corresponding to upper and lower jaws, is evidence of the bite of medium-sized mosasaurs. Whether this behaviour was common across all size classes of mosasaurs is not clear.
Virtually all forms were active predators of fish and ammonites; a few, such as Globidens, had blunt, spherical teeth, specialized for crushing mollusk shells. The smaller genera, such as Platecarpus and Dallasaurus, which were about 1–6 m (3.3–19.7 ft) long, probably fed on fish and other small prey. The smaller mosasaurs may have spent some time in fresh water, hunting for food. The larger mosasaurs, such as Tylosaurus, Hainosaurus and Mosasaurus, reached sizes of 10–15 m (33–49 ft) long and were apex predators of the Late Cretaceous oceans, attacking other marine reptiles, as well as preying on large fish and ammonites.
Soft tissue

Despite the many mosasaur remains collected worldwide, knowledge of the nature of their skin coverings remains in its early stages. Few mosasaurid specimens collected from around the world retain fossilized scale imprints. This lack may be due to the delicate nature of the scales, which nearly eliminates the possibility of preservation, in addition to the preservation sediment types and the marine conditions under which the preservation occurred. Until the discovery of several mosasaur specimens with remarkably well-preserved scale imprints from late Maastrichtian deposits of the Muwaqqar Chalk Marl Formation of Harrana[9] in Jordan, knowledge of the nature of mosasaur integument was mainly based on very few accounts describing early mosasaur fossils dating back to the upper Santonian–lower Campanian, such as the famous Tylosaurus specimen (KUVP-1075) from Gove County, Kansas.[10] Material from Jordan has shown that the bodies of mosasaurs, as well as the membranes between their fingers and toes, were covered with small, overlapping, diamond-shaped scales resembling those of snakes. Much like those of modern reptiles, mosasaur scales varied across the body in type and size. In Harrana specimens, two types of scales were observed on a single specimen: keeled scales covering the upper regions of the body and smooth scales covering the lower.[9] As ambush predators, lurking and quickly capturing prey using stealth tactics,[11] they may have benefited from the nonreflective, keeled scales.[9] Additionally, mosasaurs had large pectoral girdles, and such genera as Plotosaurus may have used their front flippers in a breaststroke motion to gain added bursts of speed during an attack on prey.[12]
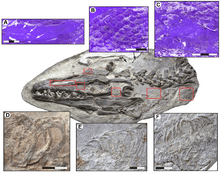
More recently, a fossil of Platecarpus tympaniticus has been found that preserved not only skin impressions, but also internal organs. Several reddish areas in the fossil may represent the heart, lungs, and kidneys. The trachea is also preserved, along with part of what may be the retina in the eye. The placement of the kidneys is farther forward in the abdomen than it is in monitor lizards, and is more similar to those of cetaceans. As in cetaceans, the bronchi leading to the lungs run parallel to each other instead of splitting apart from one another as in monitors and other terrestrial reptiles. In mosasaurs, these features may be internal adaptations to fully marine lifestyles.[5]
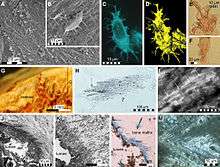
In 2011, collagen protein was recovered from a Prognathodon humerus dated to the Cretaceous.[13]
In 2005, a case study by A.S. Schulp, E.W.A Mulder, and K. Schwenk outlined the fact that mosasaurs had paired fenestrae in their palates. In monitor lizards and snakes, paired fenestrae are associated with a forked tongue, which is flicked in and out to detect chemical traces and provide a directional sense of smell. They therefore proposed that mosasaurs probably also had a sensitive forked tongue.[14]
Metabolism
A study published in 2016 by T. Lyn Harrell, Alberto Pérez-Huerta and Celina Suarez showed that mosasaurs were endothermic. The study contradicted findings published in 2010 indicating mosasaurs were ectothermic. The 2010 study did not use warm-blooded animals for comparison but analogous groups of common marine animals. Based on comparisons with modern warm-blooded animals and fossils of known cold-blooded animals from the same time period, the 2016 study found mosasaurs likely had body temperatures similar to those of contemporary seabirds and were able to internally regulate their temperatures to remain warmer than the surrounding water.[15]
Coloration
The coloration of mosasaurs was unknown until 2014, when the findings of Johan Lindgren of Lund University and colleagues revealed the pigment melanin in the fossilized scales of a mosasaur. Mosasaurs were likely countershaded, with dark backs and light underbellies, much like a great white shark or leatherback sea turtle, the latter of which had fossilized ancestors for which color was also determined. The findings were described in the journal Nature.[16]
Ontogeny and growth
Mosasaur growth is not well understood, as specimens of juveniles are rare, and many were mistaken for hesperornithine birds when discovered 100 years ago. However, the discovery of several specimens of juvenile and neonate-sized mosasaurs unearthed more than a century ago indicate that mosasaurs gave birth to live young, and that they spent their early years of life out in the open ocean, not in sheltered nurseries or areas such as shallow water as previously believed. Whether mosasaurs provided parental care, like other marine reptiles such as plesiosaurs, is currently unknown. The discovery of young mosasaurs was published in the journal Palaeontology.[17]
In late 2014 the Guinness World Records awarded the museum with a record for Largest Publicly Displayed Mosasaur - Bruce. The record was added to the 2016 print edition of the Guinness World Records.[18]
Possible Eggs
A 2020 study published in Nature described a large fossilized hatched egg from Antarctica from the very end of the Cretaceous, about 68 million years ago. The egg is considered one of the largest amniote eggs ever known, rivalling that of the elephant bird, and due to its soft, thin, folded texture, it likely belonged to a marine animal. While the organism that produced it remains unknown, the egg's pore structure is very similar to that of extant lepidosaurs such as lizards and snakes, and presence of mosasaur fossils nearby indicates that it may have been a mosasaur egg. It is unknown whether the egg was laid on land or in the water. The egg was assigned to the newly-described oospecies Antarcticoolithus bradyi.[19][20][21]
Environment
Paleotologists compared the taxonomic diversity and patterns of morphological disparity in mosasaurs with sea level, sea surface temperature, and stable carbon isotope curves for the Upper Cretaceous to explore factors that may have influenced their evolution. No single factor unambiguously accounts for all radiations, diversification, and extinctions; however, the broader patterns of taxonomic diversification and morphological disparity point to niche differentiation in a “fishing up” scenario under the influence of “bottom-up” selective pressures. The most likely driving force in mosasaur evolution was high productivity in the Late Cretaceous, driven by tectonically controlled sea levels and climatically controlled ocean stratification and nutrient delivery. When productivity collapsed at the end of the Cretaceous, coincident with bolide impact, mosasaurs became extinct.[22]

Sea levels were high during the Cretaceous period, causing marine transgressions in many parts of the world, and a great inland seaway in what is now North America. Mosasaur fossils have been found in the Netherlands, Belgium, Denmark, Portugal, Sweden, South Africa, Spain, France, Germany, Poland, the Czech Republic,[23] Bulgaria, the United Kingdom,[24][25] Russia, Ukraine, Kazakhstan, Azerbaijan,[26] Japan,[27] Egypt, Israel, Jordan, Syria,[28] Turkey,[29] Niger,[30][31] Angola, Morocco, Australia, New Zealand, and on Vega Island off the coast of Antarctica. Tooth taxon Globidens timorensis is known from the island of Timor; however, the phylogenetic placement of this species is uncertain and it might not even be a mosasaur.[32] Mosasaurs have been found in Canada in Manitoba and Saskatchewan[33] and in much of the contiguous United States. Complete or partial specimens have been found in Alabama, Mississippi, New Jersey, Tennessee, and Georgia, as well as in states covered by the Cretaceous seaway: Texas, southwest Arkansas, New Mexico, Kansas,[34] Colorado, Nebraska, South Dakota, Montana, Wyoming, and the Pierre Shale/Fox Hills formations of North Dakota.[35] Lastly, mosasaur bones and teeth are also known from California, Mexico, Colombia,[36] Brazil,[28] Peru, and Chile.[37]
Many of the so-called 'dinosaur' remains found on New Zealand are actually mosasaurs and plesiosaurs , both being Mesozoic predatory marine reptiles.
Discovery

The first publicized discovery of a partial fossil mosasaur skull in 1764 by quarry workers in a subterranean gallery of a limestone quarry in Mount Saint Peter, near the Dutch city of Maastricht, preceded any major dinosaur fossil discoveries, but remained little known. However, a second find of a partial skull drew the Age of Enlightenment's attention to the existence of fossilized animals that were different from any known living creatures. When the specimen was discovered between 1770 and 1774, Johann Leonard Hoffmann, a surgeon and fossil collector, corresponded about it with the most influential scientists of his day, making the fossil famous. The original owner, though, was Godding, a canon of Maastricht cathedral.
When the French revolutionary forces occupied Maastricht in 1794, the carefully hidden fossil was uncovered, after a reward, it is said, of 600 bottles of wine, and transported to Paris. After it had been earlier interpreted as a fish, a crocodile, and a sperm whale, the first to understand its lizard affinities was the Dutch scientist Adriaan Gilles Camper in 1799. In 1808, Georges Cuvier confirmed this conclusion, although le Grand Animal fossile de Maëstricht was not actually named Mosasaurus ('Meuse reptile') until 1822 and not given its full species name, Mosasaurus hoffmannii, until 1829. Several sets of mosasaur remains, which had been discovered earlier at Maastricht but were not identified as mosasaurs until the 19th century, have been on display in the Teylers Museum, Haarlem, procured from 1790.
The Maastricht limestone beds were rendered so famous by the mosasaur discovery, they have given their name to the final six-million-year epoch of the Cretaceous, the Maastrichtian.
Relationships
Cladogram of the Mosasauridae modified from Simões et al. (2017):[38]
| Mosasauridae |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Evolutionary history



Based on features such as the double row of pterygoid ("flanged") teeth on the palate, the loosely hinged jaw, modified/reduced limbs and probable methods of locomotion, many researchers believe that snakes share a common marine ancestry with mosasaurs, a suggestion advanced in 1869 by Edward Drinker Cope, who coined the term Pythonomorpha to unite them. The idea lay dormant for more than a century, to be revived in the 1990s.[39][40] Recently, the discovery of Najash rionegrina, a fossorial snake from South America, cast doubt on the marine origin hypothesis.
The skeleton of Dallasaurus turneri, described by Bell and Polcyn (2005), has a mixture of features present in the skeletons of derived mosasaurs and in the skeletons of mosasaurid ancestors, such as aigialosaurids. Dallasaurus retains facultatively terrestrial limbs similar in their structure to the limbs of aigialosaurids and terrestrial squamates (plesiopedal limb condition), unlike derived mosasaurids, which evolved paddle-like limbs (hydropedal limb condition). However, the skeleton of Dallasaurus simultaneously had several characters that linked it with derived members of the subfamily Mosasaurinae; the authors of its description listed "invasion of the parietal by medial tongues from the frontal, teeth with smooth medial enamel surface, high coronoid buttress on surangular, interdigitate anterior scapulo-coracoid suture, humeral postglenoid process, elongate atlas synapophysis, sharp anterodorsal ridge on synapophyses, vertically oriented vertebral condyles, elongate posterior thoracic vertebrae, and fused haemal arches" as the characters uniting Dallasaurus with Mosasaurinae.[41] The phylogenetic analysis conducted by Bell and Polcyn indicated that hydropedal mosasaurids did not form a clade that wouldn't also include plesiopedal taxa, such as Dallasaurus, Yaguarasaurus, Russellosaurus, Tethysaurus, Haasiasaurus and Komensaurus (in 2005 only informally known as "Trieste aigialosaur"); the analysis indicated that hydropedal limb condition evolved independently in three different groups of mosasaurs (Halisaurinae, Mosasaurinae and the group containing the subfamilies Tylosaurinae and Plioplatecarpinae).[41][42] The result of this phylogenetic study was subsequently mostly confirmed by the analyses conducted by Caldwell and Palci (2007) and Leblanc, Caldwell and Bardet (2012);[43][44] the analysis conducted by Makádi, Caldwell and Ősi (2012) indicated that hydropedal limb condition evolved independently in two groups of mosasaurs (in Mosasaurinae and in the clade containing Halisaurinae, Tylosaurinae and Plioplatecarpinae).[45] Conrad et al. (2011), on the other hand, recovered hydropedal mosasaurs forming a clade that excluded their plesiopedal relatives.[46] If the hypothesis of Bell and Polcyn (2005) is correct, then mosasaurs in the traditional sense of the word, i.e. "lizards that evolved paddle-like limbs and radiated into aquatic environments in the late Mesozoic, going extinct at the end of that era",[42] are actually polyphyletic; Bell and Polcyn (2005) maintained monophyletic Mosasauridae by including Dallasaurus and other aforementioned plesiopedal taxa in the family as well,[41] while Caldwell (2012) suggested (though explicitly stated that it was not "a formal proposal of new nomenclature") to restrict Mosasauridae only to the genus Mosasaurus and its closest hydropedal relatives.[42]
The exact phylogenetic position of the clade containing mosasaurids and their closest relatives (aigialosaurids and dolichosaurs) within Squamata remains uncertain. Some cladistic analyses recovered them as the closest relatives of snakes,[47][48] taking into account similarities in jaw and skull anatomies;[47] however, this has been disputed[49][50][51] and the morphological analysis conducted by Conrad (2008) recovered them as varanoids closely related to terrestrial monitor lizards instead.[49] Subsequent analysis of anguimorph relationships conducted by Conrad et al. (2011) based on morphology alone recovered mosasaurids, aigialosaurids and dolichosaurs as anguimorphs lying outside the least inclusive clade containing monitor lizards and helodermatids; the analysis based on combined datasets of morphological and molecular data, on the other hand, found them more closely related to monitor lizards and the earless monitor lizard than to helodermatids and the Chinese crocodile lizard.[46] The large morphological analysis conducted by Gauthier et al. (2012) recovered mosasaurids, aigialosaurids and dolichosaurids in an unexpected position as basal members of the clade Scincogekkonomorpha (containing all taxa sharing a more recent common ancestor with Gekko gecko and Scincus scincus than with Iguana iguana[49]) that didn't belong to the clade Scleroglossa. The phylogenetic position of these taxa turned out to be highly dependent on which taxa were included in or excluded from the analysis. When mosasaurids were excluded from the analysis, dolichosaurs and aigialosaurids were recovered within Scleroglossa, forming a sister group to the clade containing snakes, amphisbaenians, dibamids and the American legless lizard. When mosasaurids were included in the analysis, and various taxa with reduced or absent limbs other than snakes (such as dibamids or amphisbaenians) were excluded, mosasaurids, aigialosaurids and dolichosaurs were recovered inside Scleroglossa forming the sister group to snakes.[52] Longrich, Bhullar and Gauthier (2012) conducted a morphological analysis of squamate relationships using a modified version of the matrix from the analysis of Gauthier et al. (2012); they found the phylogenetic position of the clade containing mosasaurs and their closest relatives within Squamata to be highly unstable, with the clade "variously being recovered outside Scleroglossa (as in Gauthier et al., 2012) or alongside the limbless forms".[53]
Distribution
Though no individual genus or subfamily is found worldwide, the Mosasauridae as a whole achieved global distribution during the Late Cretaceous with many locations typically having complex mosasaur faunas with multiple different genera and species in different ecological niches.
Two African countries are particularly rich in mosasaurs: Morocco[54] and Angola.[55][56]
References
- Dash, Sean (2008). Prehistoric Monsters Revealed. United States: Workaholic Productions / History Channel. Retrieved December 18, 2015.
- {{ the cite of journal |title=Pelagic neonatal fossils support viviparity and precocial life history of Cretaceous mosasaurs |first1=Daniel J. |last1=Field |first2=Aaron |last2=LeBlanc |first3=Adrienne |last3=Gau1 |first4=Adam D. |last4=Behlke |journal=Palaeontology |date=10 April 2015 |doi=10.1111/pala.12165 |volume=58 |issue =3|pages=401–407|url=https://semanticscholar.org/paper/0edb960fae376ab1aee327361cd227fcbd6d1452 }}
- Grigoriev, D.W. (2014). "Giant Mosasaurus hoffmanni (Squamata, Mosasauridae) from the Late Cretaceous (Maastrichtian) of Penza, Russia" (PDF). Proceedings of the Zoological Institute RAS. 318 (2): 148–167. Retrieved 26 June 2016.
- "Largest mosasaur on display". Guinness World Records. 2014. Retrieved 27 June 2016.
- Lindgren, J.; Caldwell, M.W.; Konishi, T.; Chiappe, L.M. (2010). Farke, Andrew Allen (ed.). "Convergent Evolution in Aquatic Tetrapods: Insights from an Exceptional Fossil Mosasaur". PLoS ONE. 5 (8): e11998. Bibcode:2010PLoSO...511998L. doi:10.1371/journal.pone.0011998. PMC 2918493. PMID 20711249.
- Lindgren, J.; Kaddumi, H. F.; Polcyn, M. J. (2013). "Soft tissue preservation in a fossil marine lizard with a bilobed tail fin". Nature Communications. 4: 2423. Bibcode:2013NatCo...4.2423L. doi:10.1038/ncomms3423. PMID 24022259.
- Osborn, Henry Fairfield (1899). "A Complete Mosasaur Skeleton, Osseous and Cartilaginous". Memoirs of the American Museum of Natural History. 1 (4): 167–188. Bibcode:1899Sci....10..919O. doi:10.1126/science.10.260.919. hdl:2027/mdp.39015042532336. Retrieved 25 November 2014.
- Everhart, Mike (13 January 2013). "Origin of the Dorsal Fringe on Mosasaurs". Oceans of Kansas. Retrieved 25 November 2014.
- Kaddumi, H.F. (2009). "On the latest scale coverings of mosasaurs (Squamata: Mosasauridae) from the Harrana Fauna in addition to the description of s new species of Mosasaurus". Fossils of the Harrana Fauna and the Adjacent Areas. Amman: Eternal River Museum of Natural History. pp. 80–94.
- Snow, F. H. (1878). "On the dermal covering of a mosasauroid reptile". Transactions of the Kansas Academy of Science. 6: 54–58. doi:10.2307/3623557. JSTOR 3623557.
- Massare, J. A. (1987). "Tooth morphology and prey preference of Mesozoic marine reptiles". Journal of Vertebrate Paleontology. 7 (2): 121–137. doi:10.1080/02724634.1987.10011647.
- "Did mosasaurs do the breast stroke?".
- Lindgren, Johan; Uvdal, Per; Engdahl, Anders; Lee, Andrew H.; Alwmark, Carl; Bergquist, Karl-Erik; Nilsson, Einar; Ekström, Peter; Rasmussen, Magnus; Douglas, Desirée A.; Polcyn, Michael J.; Jacobs, Louis L. (29 April 2011). "Microspectroscopic Evidence of Cretaceous Bone Proteins". PLoS ONE. 6 (4): e19445. Bibcode:2011PLoSO...619445L. doi:10.1371/journal.pone.0019445. ISSN 1932-6203. PMC 3084868. PMID 21559386.
- Schulp, A. S.; Mulder, E. W. A.; Schwenk, K. (2005-09-01). "Did mosasaurs have forked tongues?". Netherlands Journal of Geosciences. 84 (3): 359–371. doi:10.1017/S0016774600021144.
- Harrell, T. Lynn; Pérez-Huerta, Alberto; Suarez, Celina A.; Benson, Roger (May 2016). "Endothermic mosasaurs? Possible thermoregulation of Late Cretaceous mosasaurs (Reptilia, Squamata) indicated by stable oxygen isotopes in fossil bioapatite in comparison with coeval marine fish and pelagic seabirds". Palaeontology. 59 (3): 351–363. doi:10.1111/pala.12240. Lay summary – ScienceDaily (May 6, 2016).
- Lindgren, J.; Sjövall, P.; Carney, R. M.; Uvdal, P.; Gren, J. A.; Dyke, G.; Schultz, B. P.; Shawkey, M. D.; Barnes, K. R.; Polcyn, M. J. (2014). "Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles". Nature. 506 (7489): 484–8. Bibcode:2014Natur.506..484L. doi:10.1038/nature12899. PMID 24402224.
- "What life was like for newborn giant sea lizards during the age of the dinosaur". ScienceDaily. Retrieved 2017-08-01.
- "Largest mosasaur on display". Guinness World Records. Retrieved 2020-06-03.
- "Mystery egg likely 'belonged to giant sea reptile'". BBC News. 2020-06-17. Retrieved 2020-06-18.
- Joel, Lucas (2020-06-17). "Life Hatched From Soft Eggs, Some a Foot Long, in Dinosaur Era". The New York Times. ISSN 0362-4331. Retrieved 2020-06-18.
- Legendre, Lucas J.; Rubilar-Rogers, David; Musser, Grace M.; Davis, Sarah N.; Otero, Rodrigo A.; Vargas, Alexander O.; Clarke, Julia A. (2020-06-17). "A giant soft-shelled egg from the Late Cretaceous of Antarctica". Nature: 1–4. doi:10.1038/s41586-020-2377-7. ISSN 1476-4687.
- Polcyn, M. J.; Jacobs, L. L.; Araujo, R.; Schulp, A. S.; Mateus, O. (2014). "Physical drivers of mosasaur evolution". Palaeogeography, Palaeoclimatology, Palaeoecology. 400: 17–27. Bibcode:2014PPP...400...17P. doi:10.1016/j.palaeo.2013.05.018.
- "Druhohorní plazi v Čechách II". DinosaurusBlog. 2015-07-13. Retrieved 2017-08-01.
- "St. James' Pit, Norwich (SSSI)" (PDF). Natural England. 2014. Retrieved 25 November 2014.
- Jagt, John W. M.; Motchurova-Dekova, Neda; Ivanov, Plamen; Cappetta, Henri; Schulp, Anne S. (2006). "Latest Cretaceous mosasaurs and lamniform sharks from Labirinta cave, Vratsa District (northwest Bulgaria): A preliminary note". Geoloski Anali Balkanskoga Poluostrva. 67 (67): 51–63. doi:10.2298/gabp0667051j.
- Storrs, Glenn W.; Arkhangelskii, Maxim S.; Efimov, Vladimir M. (2000). "Mesozoic marine reptiles of Russia and other former Soviet republics". In Benton, M. J.; Shishkin, M. A.; Unwin, D. M. (eds.). The age of dinosaurs in Russia and Mongolia. Cambridge: Cambridge University Press. pp. 187–210. ISBN 978-0521554763.
- Konishi, Takuya; Tanimoto, Masahiro; Utsunomiya, Satoshi; Sato, Masahiro; Watanabe, Katsunori (2012). "A Large Mosasaurine (Squamata: Mosasauridae) from the Latest Cretaceous of Osaka Prefecture (Sw Japan)". Paleontological Research. 16 (2): 79–87. doi:10.2517/1342-8144-16.2.079.
- Bardet, Nathalie; Pereda Suberbiola, Xabier; Iarochène, Mohamed; Amalik, Mohamed; Bouya, Baadi (Sep 2005). "Durophagous Mosasauridae (Squamata) from the Upper Cretaceous phosphates of Morocco, with description of a new species of Globidens". Netherlands Journal of Geosciences. 84 (3): 167–175. doi:10.1017/S0016774600020953.
- Bardet, Nathalie; Tunoğlu, Cemal (19 September 2002) [24 Aug 2010]. "The first mosasaur (Squamata) from the Late Cretaceous of Turkey". Journal of Vertebrate Paleontology. 22 (3): 712–715. doi:10.1671/0272-4634(2002)022[0712:TFMSFT]2.0.CO;2. ISSN 0272-4634.
- Lingham-Soliar, Theagarten (1991). "Mosasaurs from the upper Cretaceous of Niger". Palaeontology. 34 (3): 653–670 – via BioStor.
- Lingham-Soliar, Theagarten (1998). "A new mosasaur Pluridens walkeri from the Upper Cretaceous, Maastrichtian of the Iullemmeden Basin, southwest Niger". Journal of Vertebrate Paleontology. 18 (4): 709–717. doi:10.1080/02724634.1998.10011100.
- Martin, James E. (2007). "A new species of the durophagous mosasaur, Globidens (Squamata: Mosasauridae) from the Late Cretaceous Pierre Shale Group of central South Dakota, USA". In Martin, James E.; Parris, David C. (eds.). The Geology and Paleontology of the Late Cretaceous Marine Deposits of the Dakotas. 427. The Geological Society of America. pp. 177–198. doi:10.1130/2007.2427(13). ISBN 978-0-8137-2427-0.
- "General Information". Canadian Fossil Discovery Centre. 2014. Retrieved 25 November 2014.
- Michael J. Everhart (2005). "Chapter 9: Enter the Mosasaurs". Oceans of Kansas: a natural history of the western interior sea. Bloomington: Indiana University Press. ISBN 978-0-253-34547-9.
- Getman, Myron (1994). Occurrences of Mosasaur and other reptilian fossil remains from the Fox Hills Formation (Maastrichtian: late Cretaceous) of North Dakota (Geology Honors thesis). St. Lawrence University Dept. of Geology.
- Páramo-Fonseca, María Eurídice (1 March 2012). "Mosasauroids from Colombia". Bulletin de la Société Géologique de France. 183 (2): 103–109. doi:10.2113/gssgfbull.183.2.103. ISSN 0037-9409 – via GeoScienceWorld.
- Otero, Rodrigo A.; Parham, James F.; Soto-Acuña, Sergio; Jimenez-Huidobro, Paulina; Rubilar-Rogers, David (2012). "Marine reptiles from Late Cretaceous (early Maastrichtian) deposits in Algarrobo, central Chile". Cretaceous Research. 35: 124–132. doi:10.1016/j.cretres.2011.12.003.
- Simões, Tiago R.; Vernygora, Oksana; Paparella, Ilaria; Jimenez-Huidobro, Paulina; Caldwell, Michael W. (2017-05-03). "Mosasauroid phylogeny under multiple phylogenetic methods provides new insights on the evolution of aquatic adaptations in the group". PLOS ONE. 12 (5): e0176773. Bibcode:2017PLoSO..1276773S. doi:10.1371/journal.pone.0176773. ISSN 1932-6203. PMC 5415187. PMID 28467456.
- "Palaeos Vertebrates Squamata: Pythonomorpha". palaeos.com. 2012. Retrieved 25 November 2014.
- Everhart, M. J. (2000). "Mosasaurs: Last of the Great Marine Reptiles". Prehistoric Times (44): 29–31. Retrieved 25 November 2014.
- Bell, G. L.; Polcyn, M. J. (Sep 2005). "Dallasaurus turneri, a new primitive mosasauroid from the Middle Turonian of Texas and comments on the phylogeny of Mosasauridae (Squamata)". Netherlands Journal of Geosciences. 84 (3): 177–194. doi:10.1017/S0016774600020965.
- Caldwell, Michael W. (2012-01-01). "A challenge to categories: "What, if anything, is a mosasaur?"". Bulletin de la Société Géologique de France. 183 (1): 7–34. doi:10.2113/gssgfbull.183.1.7. ISSN 0037-9409.
- Leblanc, Aaron R. H.; Caldwell, Michael W.; Bardet, Nathalie (January 2012). "A new mosasaurine from the Maastrichtian (Upper Cretaceous) phosphates of Morocco and its implications for mosasaurine systematics". Journal of Vertebrate Paleontology. 32 (1): 82–104. doi:10.1080/02724634.2012.624145. ISSN 0272-4634.
- Caldwell, Michael W.; Palci, Alessandro (2007-12-12). "A new basal mosasauroid from the Cenomanian (U. Cretaceous) of Slovenia with a review of mosasauroid phylogeny and evolution". Journal of Vertebrate Paleontology. 27 (4): 863–880. doi:10.1671/0272-4634(2007)27[863:ANBMFT]2.0.CO;2. ISSN 0272-4634.
- Makádi, L. S.; Caldwell, M. W.; Ősi, A. (2012) [12 Jan 2012]. Butler, Richard J (ed.). "The First Freshwater Mosasauroid (Upper Cretaceous, Hungary) and a New Clade of Basal Mosasauroids". PLoS ONE. 7 (12): e51781. Bibcode:2012PLoSO...751781M. doi:10.1371/journal.pone.0051781. PMC 3526648. PMID 23284766.
- Conrad, Jack L.; Ast, Jennifer C.; Montanari, Shaena; Norell, Mark A. (22 July 2010). "A combined evidence phylogenetic analysis of Anguimorpha (Reptilia: Squamata)". Cladistics. 27 (3): 230–277. doi:10.1111/j.1096-0031.2010.00330.x. ISSN 0748-3007.
- Lee, Michael S. Y. (29 January 1997). "The phylogeny of varanoid lizards and the affinities of snakes". Philosophical Transactions of the Royal Society of London B: Biological Sciences. 352 (1349): 53–91. Bibcode:1997RSPTB.352...53L. doi:10.1098/rstb.1997.0005. ISSN 0962-8436. PMC 1691912.
- Lee, Michael S. Y. (22 June 2005). "Molecular evidence and marine snake origins". Biology Letters. 1 (2): 227–230. doi:10.1098/rsbl.2004.0282. ISSN 1744-9561. PMC 1626205. PMID 17148173.
- Conrad, Jack L. (3 June 2008). "Phylogeny And Systematics Of Squamata (Reptilia) Based On Morphology". Bulletin of the American Museum of Natural History. 310: 1–182. doi:10.1206/310.1. ISSN 0003-0090.
- Vidal, Nicolas; Hedges, S. Blair (7 May 2004). "Molecular evidence for a terrestrial origin of snakes". Proceedings of the Royal Society of London B: Biological Sciences. 271 (Suppl 4): S226–S229. doi:10.1098/rsbl.2003.0151. ISSN 0962-8452. PMC 1810015. PMID 15252991.
- Apesteguía, Sebastián; Zaher, Hussam (April 2006). "A Cretaceous terrestrial snake with robust hindlimbs and a sacrum". Nature. 440 (7087): 1037–1040. Bibcode:2006Natur.440.1037A. doi:10.1038/nature04413. ISSN 0028-0836. PMID 16625194.
- Gauthier, Jacques A.; Kearney, Maureen; Maisano, Jessica Anderson; Rieppel, Olivier; Behlke, Adam D.B. (April 2012). "Assembling the Squamate Tree of Life: Perspectives from the Phenotype and the Fossil Record". Bulletin of the Peabody Museum of Natural History. 53 (1): 3–308. doi:10.3374/014.053.0101. ISSN 0079-032X.
- Longrich, Nicholas R.; Bhullar, Bhart-Anjan S.; Gauthier, Jacques A. (26 December 2012). "Mass extinction of lizards and snakes at the Cretaceous–Paleogene boundary". Proceedings of the National Academy of Sciences. 109 (52): 21396–21401. Bibcode:2012PNAS..10921396L. doi:10.1073/pnas.1211526110. ISSN 0027-8424. PMC 3535637. PMID 23236177.
- Bardet, Nathalie; Pereda Suberbiola, Xabier; Iarochene, Mohamed; Bouyahyaoui, Fatima; Bouya, Baadi; Amaghzaz, Mbarek (May 2004). "Mosasaurus beaugei Arambourg, 1952 (Squamata, Mosasauridae) from the Late Cretaceous phosphates of Morocco". Geobios. 37 (3): 315–324. doi:10.1016/j.geobios.2003.02.006.
- Polcyn, Michael J.; Jacobs, Louis L.; Schulp, Anne S.; Mateus, Octávio (March 2010). "The North African Mosasaur Globidens phosphaticus from the Maastrichtian of Angola". Historical Biology. 22 (1–3): 175–185. doi:10.1080/08912961003754978.
- Mateus, Octávio; Callapez, Pedro M.; Polcyn, Michael J.; Schulp, Anne S.; Gonçalves, António Olímpio; Jacobs, Louis L. (2019). "The Fossil Record of Biodiversity in Angola Through Time: A Paleontological Perspective". Biodiversity of Angola: Science & Conservation: A Modern Synthesis. Springer International Publishing. pp. 53–76. doi:10.1007/978-3-030-03083-4_4. ISBN 978-3-030-03082-7.
External links
| Wikimedia Commons has media related to Mosasauridae. |
| Wikispecies has information related to Mosasauridae |
- Palaeos: Vertebrates: Mosasaurs
- BBC Science and Nature: Mosasaurs
- Mike Everhart and David Lewis, "Mesozoic marine monsters of the Mangahouanga": New Zealand fossil fauna
- Mike Everhart, "A day in the life of a Mosasaur": life in the Sea of Kansas, illus. by Carl Buell
- Mike Everhart, "Mosasaurus hoffmani" until 1829.
- Mosasaurus maximus mounted skeleton at University of Texas Memorial Museum
- Canadian Fossil Discovery Centre
- "The Mosasaur of Maastricht" by Hennie Reuvers in Crossroads web magazine
- "Mosasaurs terrorized Cretaceous rivers" Planet Earth online
- Georgia Southern University Museum Mosasaur Exhibit
- Kansas Geological Survey Vol IV (1899), containing the famous summary of American mosasaurs by Samuel Williston.
- William R. Wahl * MOSASAUR BITE MARKS ON AN AMMONITE. PRESERVATION OF AN ABORTED ATTACK?
- Mosasaur diet