Medicinal fungi
Medicinal fungi are fungi which contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or are under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.
History
Although fungi products have long been used in traditional medicine, the ability to identify beneficial properties and then extract the active ingredient started with the discovery of penicillin by Alexander Fleming in 1928.[1] Since that time, many potential antibiotics were discovered and the potential for various fungi to synthesize biologically active molecules useful in various clinical therapies has been under research. Pharmacological research identified antifungal, antiviral, and antiprotozoan compounds from fungi.[2]
Ganoderma lucidum, known in Chinese as líng zhī ("spirit plant"), and in Japanese as mannentake ("10,000-year mushroom"), has been well studied. Another species of genus Ganoderma, G. applanatum, remains under basic research. Inonotus obliquus was used in Russia as early as the 16th century, and it featured in Alexandr Solzhenitsyn's 1967 novel Cancer Ward.[3]
Research and drug development
Cancer
Paclitaxel is synthesised using Penicillium raistrickii and plant cell fermentation. Fungi can synthesize other mitotic inhibitors including vinblastine, vincristine, podophyllotoxin, griseofulvin, aurantiamine, oxaline, and neoxaline.[6][7]
11,11'-Dideoxyverticillin A, an isolate of marine Penicillium, was used to create dozens of semi-synthetic, candidate anticancer compounds.[8] 11,11'-Dideoxyverticillin A, andrastin A, barceloneic acid A, and barceloneic acid B, are farnesyl transferase inhibitors that can be made by Penicillium.[9] 3-O-Methylfunicone, anicequol, duclauxin, and rubratoxin B, are anticancer/cytotoxic metabolites of Penicillium.
Penicillium is a potential source of the leukemia medicine asparaginase.[10]
Some countries have approved Beta-glucan fungal extracts lentinan, polysaccharide-K, and polysaccharide peptide as immunologic adjuvants.[11] Evidence suggests this use as effective in prolonging and improving the quality of life for patients with certain cancers, although the Memorial Sloan-Kettering Cancer Center observes that "well designed, large scale studies are needed to establish the role of lentinan as a useful adjunct to cancer treatment".[12] According to Cancer Research UK, "there is currently no evidence that any type of mushroom or mushroom extract can prevent or cure cancer".[13] Fungal metabolites such as ergosterol, clavilactones, and triterpenoids are efficient Cdk inhibitors that lead to G1/S or G2/M arrest of cancer cells. Other metabolites, such as panepoxydone, are inhibitors of NF-κB. Fucose and mannose fragments of fungal cell wall are antagonists of VEGF-receptors [14]
Antibacterial agents (antibiotics)
Alexander Fleming led the way to the beta-lactam antibiotics with the Penicillium mold and penicillin. Subsequent discoveries included alamethicin, aphidicolin, brefeldin A, Cephalosporin, cerulenin, citromycin, eupenifeldin, fumagillin, fusafungine, fusidic acid, itaconic acid, MT81, nigrosporin B, usnic acid, verrucarin A, vermiculine and many others.
Antibiotics retapamulin, tiamulin, and valnemulin are derivatives of the fungal metabolite pleuromutilin. Plectasin, austrocortilutein, austrocortirubin, coprinol, oudemansin A, strobilurin, illudin, pterulone, and sparassol are under research for their potential antibiotic activity.
Cholesterol biosynthesis inhibitors

Statins are an important class of cholesterol-lowering drugs; the first generation of statins were derived from fungi.[15] Lovastatin, the first commercial statin, was extracted from a fermentation broth of Aspergillus terreus.[15] Industrial production is now capable of producing 70 mg lovastatin per kilogram of substrate.[16] The red yeast rice fungus, Monascus purpureus, can synthesize lovastatin, mevastatin, and the simvastatin precursor monacolin J. Nicotinamide riboside, a cholesterol biosynthesis inhibitor, is made by Saccharomyces cerevisiae.
Antifungals
Some antifungals are derived or extracted from other fungal species. Griseofulvin is derived from a number of Penicillium species, caspofungin is derived from Glarea lozoyensis.[17] Strobilurin, azoxystrobin, micafungin, and echinocandins, are all extracted from fungi. Anidulafungin is a derivative of an Aspergillus metabolite.
Antivirals
Many mushrooms have been shown to contain potential antiviral compounds remaining under preliminary research, such as: Lentinus edodes, Ganoderma lucidum, Ganoderma colossus, Hypsizygus marmoreus, Cordyceps militaris, Grifola frondosa, Scleroderma citrinum, Flammulina velutipes, and Trametes versicolor, Fomitopsis officinalis.[18][19][20][21]
Immunosuppressants
Ciclosporin, was discovered in Tolypocladium inflatum. Bredinin was discovered in Eupenicillium brefeldianum. Mycophenolic acid was discovered in Penicillium stoloniferum. Thermophilic fungi were the source of the fingolimod precursor myriocin. Aspergillus synthesizes immunosuppressants gliotoxin and endocrocin. Subglutinols are immunosuppressants isolated from Fusarium subglutinans.[22] Other compounds include mizoribine.
Malaria
Codinaeopsin, efrapeptins, zervamicins, and antiamoebin,[23] are made by fungi, and remain under basic research.
Diabetes
Many fungal isolates act as DPP-4 inhibitors, alpha-glucosidase inhibitors, and alpha amylase inhibitors in laboratory studies. Ternatin is a fungal isolate that may affect hyperglycemia.[24] Aspergillusol A is an alpha-glucosidase inhibitor made by Aspergillus. Sclerotiorin is an aldose reductase inhibitor made by Penicillium.
Psychotropic effects
Numerous fungi have well-documented psychotropic effects, some of them severe and associated with acute and life-threatening side-effects. Among these is Amanita muscaria, the fly agaric. More widely used informally are a range of fungi collectively known as "magic mushrooms", which contain psilocybin and psilocin.
The history of bread-making refers to deadly ergotism caused by ergot, most commonly Claviceps purpurea, a parasite of cereal crops. Some drugs have subsequently been extracted from ergot, including ergotamine, pergolide and cabergoline.[25]
Psychotropic compounds created from ergot alkaloids also include dihydroergotamine, methysergide, methylergometrine, hydergine, nicergoline, lisuride, bromocriptine, cabergoline, pergolide. Polyozellus multiplex synthesizes prolyl endopeptidase inhibitors polyozellin, thelephoric acid, kynapcins. Neurotrophic fungal isolates include L-theanine, tricholomalides, scabronines, termitomycesphins. Many fungi synthesize the partial, non-selective, serotonin receptor agonist/analog psilocin.
Vitamin D2
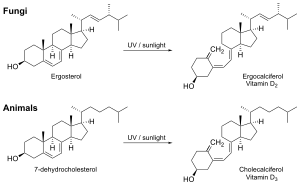
Fungi are a source of ergosterol which can be converted to vitamin D2 upon exposure to ultraviolet light.[26][27]
Edible species containing compounds of interest
Edible species contain constituents under basic research:
- Agaricus subrufescens (Agaricus blazei/brasiliensis, almond mushroom) is a fungus associated with Brazil and Japan. Blazein, a bioactive steroid, was isolated from A. subrufescens.
- The adenosine analog cordycepin was originally isolated from Cordyceps.[28] Other Cordyceps isolates include, cordymin, cordycepsidone, and cordyheptapeptide. CS-4 is commercially sold as C. sinensis, but Cs-4 has recently been confirmed to be a different species from the Cordyceps species used in traditional Chinese medicine. CS-4 is properly known as Paecilomyces hepiali. Hirsutella sinensis is the accepted asexual form of C. sinensis.
- Ganoderma lucidum (Ling zhi, mannentake, reishi) contains p-hydroxybenzoic acid, cinnamic acid, and lanostane-type triterpenoids such as ganoderic acids.
- Hydnellum peckii has yielded atromentin, a compound isolated from the mycorrhiza, and subsequently its biosynthesis has been characterized.
- Lentinula edodes (Shiitake) has been used as a source of Lentinan, AHCC, and eritadenine.[29]
- Schizophyllum commune (Split gill) has yielded schizophyllan (SPG, sizofiran, sonifilan).[30] Hydrophobins were originally isolated from S. commune. A chemically analogous polysaccharide, scleroglucan, is an isolate of Sclerotium rolfsii.
- Tolypocladium inflatum Gams yields the immunosuppressant ciclosporin.[31]
- Trametes versicolor (Coriolus versicolor, yun zhi, kawaratake, turkey tail) have produced protein-bound polysaccharides PSK and PSP (polysaccharopeptide) from different mycelia strains.[32]
- Ustilago maydis (Mexican truffle, huitlacoche, corn fungus) synthesises ustilagine and ustilagic acid.
Yeasts
Saccharomyces is used industrially to produce the amino acid lysine, as well as recombinant proteins insulin and Hepatitis B surface antigen. Transgenic yeast are used to produce artemisinin, as well as a number of insulin analogs.[33] Candida is used industrially to produce vitamins ascorbic acid and riboflavin. Pichia is used to produce the amino acid tryptophan and the vitamin pyridoxine. Rhodotorula is used to produce the amino acid phenylalanine. Moniliella is used industrially to produce the sugar alcohol erythritol.
See also
- Saccharomyces boulardii and Saccharomyces cerevisiae (baker's/brewer's yeast) extracts:
References
- "Discovery and Development of Penicillin". American Chemical Society, International Historic Chemical Landmarks. 2020. Retrieved 11 March 2020.
- Engler M, Anke T, Sterner O (1998). "Production of antibiotics by Collybia nivalis, Omphalotus olearis, a Favolaschia and a Pterula species on natural substrates". Zeitschrift für Naturforschung C. 53 (5–6): 318–24. doi:10.1515/znc-1998-5-604. PMID 9705612.
- Zheng W, Miao K, Liu Y, Zhao Y, Zhang M, Pan S, Dai Y (July 2010). "Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production". Applied Microbiology and Biotechnology. 87 (4): 1237–54. doi:10.1007/s00253-010-2682-4. PMID 20532760.
- Bemani E, Ghanati F, Rezaei A, Jamshidi M (July 2013). "Effect of phenylalanine on Taxol production and antioxidant activity of extracts of suspension-cultured hazel (Corylus avellana L.) cells". Journal of Natural Medicines. 67 (3): 446–51. doi:10.1007/s11418-012-0696-1. PMID 22847380.
- Gangadevi V, Murugan M, Muthumary J (August 2008). "Taxol determination from Pestalotiopsis pauciseta, a fungal endophyte of a medicinal plant". Sheng Wu Gong Cheng Xue Bao = Chinese Journal of Biotechnology. 24 (8): 1433–8. doi:10.1016/s1872-2075(08)60065-5. PMID 18998547.
- Nicoletti R, Ciavatta ML, Buommino E, Tufano MA (2008). "Antitumor extrolites produced by Penicillium species" (PDF). International Journal of Biomedical and Pharmaceutical Sciences. 2 (1): 1–23. Archived from the original (PDF) on 26 December 2014. Retrieved 26 August 2016.
- "Antitumor extrolites produced by Penicillium species" (PDF). Archived from the original (PDF) on December 26, 2014. Retrieved August 17, 2014. Cite journal requires
|journal=(help) - "Research update: Chemists find help from nature in fighting cancer - MIT News Office". Web.mit.edu. 2013-02-27. Retrieved 2013-12-17.
- Overy DP, Larsen TO, Dalsgaard PW, Frydenvang K, Phipps R, Munro MH, Christophersen C (November 2005). "Andrastin A and barceloneic acid metabolites, protein farnesyl transferase inhibitors from Penicillium albocoremium: chemotaxonomic significance and pathological implications". Mycological Research. 109 (Pt 11): 1243–9. doi:10.1017/S0953756205003734. PMID 16279417.
- Shrivastava A, Khan AA, Shrivastav A, Jain SK, Singhal PK (2012). "Kinetic studies of L-asparaginase from Penicillium digitatum". Preparative Biochemistry & Biotechnology. 42 (6): 574–81. doi:10.1080/10826068.2012.672943. PMID 23030468.
- Ina K, Kataoka T, Ando T (June 2013). "The use of lentinan for treating gastric cancer". Anti-Cancer Agents in Medicinal Chemistry. 13 (5): 681–8. doi:10.2174/1871520611313050002. PMC 3664515. PMID 23092289.
- "Lentinan". Memorial Sloan-Kettering Cancer Center. 27 February 2013. Retrieved 26 August 2016.
- "Mushrooms and cancer". Cancer Research UK. 2017-08-30. Retrieved 26 August 2016.
- Zmitrovich IV (2015). "Anti-cancer metabolites of Basidiomycota and their molecular targets. A review" (PDF). Vestnik Permskogo Universiteta. Biologiya. 2015 (3): 264–86.
- Tobert JA (July 2003). "Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors". Nature Reviews. Drug Discovery. 2 (7): 517–26. doi:10.1038/nrd1112. PMID 12815379.
- Jahromi MF, Liang JB, Ho YW, Mohamad R, Goh YM, Shokryazdan P (2012). "Lovastatin production by Aspergillus terreus using agro-biomass as substrate in solid state fermentation". Journal of Biomedicine & Biotechnology. 2012: 196264. doi:10.1155/2012/196264. PMC 3478940. PMID 23118499.
- Richardson MD, Warnock DW (2003-12-01). Fungal Infection Diagnosis and Management. ISBN 978-1-4051-15780.
- Pradeep P, Manju V, Ahsan MF (2019), Agrawal DC, Dhanasekaran M (eds.), "Antiviral Potency of Mushroom Constituents", Medicinal Mushrooms: Recent Progress in Research and Development, Springer Singapore, pp. 275–297, doi:10.1007/978-981-13-6382-5_10, ISBN 9789811363825
- Friedman M (November 2016). "Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans". Foods. 5 (4): 80. doi:10.3390/foods5040080. PMC 5302426. PMID 28231175.
- Zhang T, Ye J, Xue C, Wang Y, Liao W, Mao L, et al. (October 2018). "Structural characteristics and bioactive properties of a novel polysaccharide from Flammulina velutipes". Carbohydrate Polymers. 197: 147–156. doi:10.1016/j.carbpol.2018.05.069. PMID 30007599.
- Girometta C (March 2019). "Fomitopsis officinalis in the light of its bioactive metabolites: a review". Mycology. 10 (1): 32–39. doi:10.1080/21501203.2018.1536680. PMC 6394315. PMID 30834150.
- Kim H, Baker JB, Park Y, Park HB, DeArmond PD, Kim SH, et al. (August 2010). "Total synthesis, assignment of the absolute stereochemistry, and structure-activity relationship studies of subglutinols A and B". Chemistry, an Asian Journal. 5 (8): 1902–10. doi:10.1002/asia.201000147. PMID 20564278.
- Nagaraj G, Uma MV, Shivayogi MS, Balaram H (January 2001). "Antimalarial activities of peptide antibiotics isolated from fungi". Antimicrobial Agents and Chemotherapy. 45 (1): 145–9. doi:10.1128/aac.45.1.145-149.2001. PMC 90252. PMID 11120957.
- Lo HC, Wasser SP (2011). "Medicinal mushrooms for glycemic control in diabetes mellitus: history, current status, future perspectives, and unsolved problems (review)". International Journal of Medicinal Mushrooms. 13 (5): 401–26. doi:10.1615/intjmedmushr.v13.i5.10. PMID 22324407.
- Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E (January 2007). "Dopamine agonists and the risk of cardiac-valve regurgitation". The New England Journal of Medicine. 356 (1): 29–38. doi:10.1056/NEJMoa062222. PMID 17202453.
- Keegan RJ, Lu Z, Bogusz JM, Williams JE, Holick MF (January 2013). "Photobiology of vitamin D in mushrooms and its bioavailability in humans". Dermato-Endocrinology. 5 (1): 165–76. doi:10.4161/derm.23321. PMC 3897585. PMID 24494050.
- Kamweru PK, Tindibale EL (2016). "Vitamin D and Vitamin D from Ultraviolet-Irradiated Mushrooms (Review)". International Journal of Medicinal Mushrooms. 18 (3): 205–14. doi:10.1615/IntJMedMushrooms.v18.i3.30. PMID 27481154.
- Cunningham KG, Manson W, Spring FS, Hutchinson SA (December 1950). "Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link". Nature. 166 (4231): 949. Bibcode:1950Natur.166..949C. doi:10.1038/166949a0. PMID 14796634.
- Bisen PS, Baghel RK, Sanodiya BS, Thakur GS, Prasad GB (2010). "Lentinus edodes: a macrofungus with pharmacological activities". Current Medicinal Chemistry. 17 (22): 2419–30. doi:10.2174/092986710791698495. PMID 20491636.
- Mantovani G, Bianchi A, Curreli L, Ghiani M, Astara G, Lampis B, et al. (January 1997). "Clinical and immunological evaluation of schizophyllan (SPG) in combination with standard chemotherapy in patients with head and neck squamous cell carcinoma". International Journal of Oncology. 10 (1): 213–21. doi:10.3892/ijo.10.1.213. PMID 21533366.
- Borel JF, Kis ZL, Beveridge T (1995). "The history of the discovery and development of Cyclosporin (Sandimmune®)". In Merluzzi VJ, Adams J (eds.). The search for anti-inflammatory drugs case histories from concept to clinic. Boston: Birkhäuser. pp. 27–63. ISBN 978-1-4615-9846-6. Archived from the original on 2017-11-05.
- Pdq Integrative, Alternative (2002). "Medicinal Mushrooms (PDQ®): Patient Version". PDQ Cancer Information Summaries. National Cancer Institute (US). PMID 28267306.
- Peplow M. "Sanofi launches malaria drug production | Chemistry World". Rsc.org. Retrieved 2013-12-17.
External links
- Memorial Sloan-Kettering Agaricus subrufescens, Phellinus linteus, Ganoderma lucidum, Trametes versicolor and PSK, Grifola frondosa, Inonotus obliquus, Pleurotus ostreatus, Cordyceps, Shiitake, Lentinan, AHCC.
- American Cancer Society Trametes versicolor and PSK, Grifola frondosa, Shiitake.
- National Cancer Institute Shiitake, Lentinan, Cordycepin