Marine primary production
Marine primary production is the chemical synthesis in the ocean of organic compounds from atmospheric or dissolved carbon dioxide. It principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through chemosynthesis, which uses the oxidation or reduction of inorganic chemical compounds as its source of energy. Almost all life on Earth relies directly or indirectly on primary production. The organisms responsible for primary production are called primary producers or autotrophs.
| Part of a series of overviews on |
| Marine life |
|---|
|
|
Most marine primary production is generated by a diverse collection of marine microorganisms called algae and cyanobacteria. Together these form the principal primary producers at the base of the ocean food chain and produce half of the world's oxygen. Marine primary producers underpin almost all marine animal life by generating nearly all of the oxygen and food marine animals need to exist. Some marine primary producers are also ecosystem engineers which change the environment and provide habitats for other marine life.
Primary production in the ocean can be contrasted with primary production on land. Globally the ocean and the land each produce about the same amount of primary production, but in the ocean primary production comes mainly from cyanobacteria and algae, while on land it comes mainly from vascular plants.
Marine algae includes the largely invisible and often unicellular microalgae, which together with cyanobacteria form the ocean phytoplankton, as well as the larger, more visible and complex multicellular macroalgae commonly called seaweed. Seaweeds are found along coastal areas, living on the floor of continental shelves and washed up in intertidal zones. Some seaweeds drift with plankton in the sunlit surface waters (epipelagic zone) of the open ocean.
Back in the Silurian, some phytoplankton evolved into red, brown and green algae. These algae then invaded the land and started evolving into the land plants we know today. Later in the Cretaceous some of these land plants returned to the sea as mangroves and seagrasses. These are found along coasts in intertidal regions and in the brackish water of estuaries. In addition, some seagrasses, like seaweeds, can be found at depths up to 50 metres on both soft and hard bottoms of the continental shelf.
Marine primary producers
Primary producers are the autotroph organisms that make their own food instead of eating other organisms. This means primary producers become the starting point in the food chain for heterotroph organisms that do eat other organisms. Some marine primary producers are specialised bacteria and archaea which are chemotrophs, making their own food by gathering around hydrothermal vents and cold seeps and using chemosynthesis. However most marine primary production comes from organisms which use photosynthesis on the carbon dioxide dissolved in the water. This process uses energy from sunlight to convert water and carbon dioxide[2]:186–187 into sugars that can be used both as a source of chemical energy and of organic molecules that are used in the structural components of cells.[2]:1242 Marine primary producers are important because they underpin almost all marine animal life by generating most of the oxygen and food that provide other organisms with the chemical energy they need to exist.
The principal marine primary producers are cyanobacteria, algae and marine plants. The oxygen released as a by-product of photosynthesis is needed by nearly all living things to carry out cellular respiration. In addition, primary producers are influential in the global carbon and water cycles. They stabilize coastal areas and can provide habitats for marine animals. The term division has been traditionally used instead of phylum when discussing primary producers, although the International Code of Nomenclature for algae, fungi, and plants now accepts the terms as equivalent.[3]
In a reversal of the pattern on land, in the oceans, almost all photosynthesis is performed by algae and cyanobacteria, with a small fraction contributed by vascular plants and other groups. Algae encompass a diverse range of organisms, ranging from single floating cells to attached seaweeds. They include photoautotrophs from a variety of groups. Eubacteria are important photosynthetizers in both oceanic and terrestrial ecosystems, and while some archaea are phototrophic, none are known to utilise oxygen-evolving photosynthesis.[4] A number of eukaryotes are significant contributors to primary production in the ocean, including green algae, brown algae and red algae, and a diverse group of unicellular groups. Vascular plants are also represented in the ocean by groups such as the seagrasses.
Unlike terrestrial ecosystems, the majority of primary production in the ocean is performed by free-living microscopic organisms called phytoplankton. It has been estimated that half of the world's oxygen is produced by phytoplankton.[5][6] Larger autotrophs, such as the seagrasses and macroalgae (seaweeds) are generally confined to the littoral zone and adjacent shallow waters, where they can attach to the underlying substrate but still be within the photic zone. There are exceptions, such as Sargassum, but the vast majority of free-floating production takes place within microscopic organisms.
The factors limiting primary production in the ocean are also very different from those on land. The availability of water, obviously, is not an issue (though its salinity can be). Similarly, temperature, while affecting metabolic rates (see Q10), ranges less widely in the ocean than on land because the heat capacity of seawater buffers temperature changes, and the formation of sea ice insulates it at lower temperatures. However, the availability of light, the source of energy for photosynthesis, and mineral nutrients, the building blocks for new growth, play crucial roles in regulating primary production in the ocean.[7] Available Earth System Models suggest that ongoing ocean bio-geochemical changes could trigger reductions in ocean NPP between 3% and 10% of current values depending on the emissions scenario.[8]
Cyanobacteria
Cyanobacteria are a phylum (division) of bacteria, ranging from unicellular to filamentous and including colonial species, which fix inorganic carbon into organic carbon compounds. They are found almost everywhere on earth: in damp soil, in both freshwater and marine environments, and even on Antarctic rocks.[9] In particular, some species occur as drifting cells floating in the ocean, and as such were amongst the first of the phytoplankton.
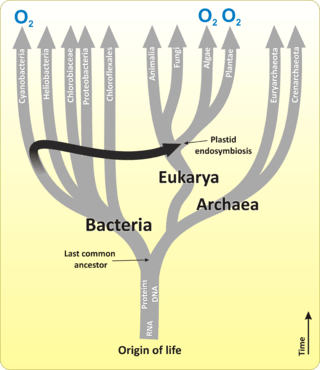
The first primary producers that used photosynthesis were oceanic cyanobacteria about 2.3 billion years ago.[10][11] The release of molecular oxygen by cyanobacteria as a by-product of photosynthesis induced global changes in the Earth's environment. Because oxygen was toxic to most life on Earth at the time, this led to the near-extinction of oxygen-intolerant organisms, a dramatic change which redirected the evolution of the major animal and plant species.[12]
.jpg)
The tiny marine cyanobacterium Prochlorococcus, discovered in 1986, forms today part of the base of the ocean food chain and accounts for more than half the photosynthesis of the open ocean[13] and an estimated 20% of the oxygen in the Earth's atmosphere.[14] It is possibly the most plentiful genus on Earth: a single millilitre of surface seawater may contain 100,000 cells or more.[15]
Originally, biologists thought cyanobacteria was algae, and referred to it as "blue-green algae". The more recent view is that cyanobacteria is a bacteria, and hence is not even in the same Kingdom as algae. Most authorities exclude all prokaryotes, and hence cyanobacteria from the definition of algae.[16][17]
Biological pigments
Biological pigments are any coloured material in plant or animal cells. All biological pigments selectively absorb certain wavelengths of light while reflecting others.[18][19] The primary function of pigments in plants is photosynthesis, which uses the green pigment chlorophyll and several colorful pigments that absorb as much light energy as possible. Chlorophyll is the primary pigment in plants; it is a chlorin that absorbs yellow and blue wavelengths of light while reflecting green. It is the presence and relative abundance of chlorophyll that gives plants their green color. Green algae and plants possess two forms of this pigment: chlorophyll a and chlorophyll b. Kelps, diatoms, and other photosynthetic heterokonts contain chlorophyll c instead of b, while red algae possess only chlorophyll a. All chlorophylls serve as the primary means plants use to intercept light in order to fuel photosynthesis.
Chloroplasts
Chloroplasts (from the Greek chloros for green, and plastes for "the one who forms"[21]) are organelles that conduct photosynthesis, where the photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it in the energy-storage molecules while freeing oxygen from water in plant and algal cells. They then use the stored energy to make organic molecules from carbon dioxide in a process known as the Calvin cycle.
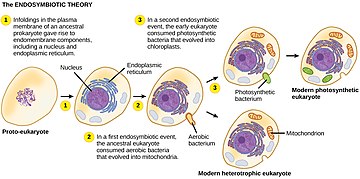
A chloroplast is a type of organelle known as a plastid, characterized by its two membranes and a high concentration of chlorophyll. They are highly dynamic—they circulate and are moved around within plant cells, and occasionally pinch in two to reproduce. Their behavior is strongly influenced by environmental factors like light color and intensity. Chloroplasts, like mitochondria, contain their own DNA, which is thought to be inherited from their ancestor—a photosynthetic cyanobacterium that was engulfed by an early eukaryotic cell.[22] Chloroplasts cannot be made by the plant cell and must be inherited by each daughter cell during cell division.
Most chloroplasts can probably be traced back to a single endosymbiotic event, when a cyanobacterium was engulfed by the eukaryote. Despite this, chloroplasts can be found in an extremely wide set of organisms, some not even directly related to each other—a consequence of many secondary and even tertiary endosymbiotic events.
Microbial rhodopsin
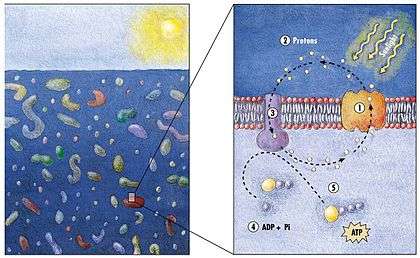
(2) it changes its configuration so a proton is expelled from the cell
(3) the chemical potential causes the proton to flow back to the cell
(4) thus generating energy
(5) in the form of adenosine triphosphate.[24]
Phototrophic metabolism relies on one of three energy-converting pigments: chlorophyll, bacteriochlorophyll, and retinal. Retinal is the chromophore found in rhodopsins. The significance of chlorophyll in converting light energy has been written about for decades, but phototrophy based on retinal pigments is just beginning to be studied.[25]
.jpg)
In 2000 a team of microbiologists led by Edward DeLong made a crucial discovery in the understanding of the marine carbon and energy cycles. They discovered a gene in several species of bacteria[27][28] responsible for production of the protein rhodopsin, previously unheard of in bacteria. These proteins found in the cell membranes are capable of converting light energy to biochemical energy due to a change in configuration of the rhodopsin molecule as sunlight strikes it, causing the pumping of a proton from inside out and a subsequent inflow that generates the energy.[29] The archaeal-like rhodopsins have subsequently been found among different taxa, protists as well as in bacteria and archaea, though they are rare in complex multicellular organisms.[30][31][32]
Research in 2019 shows these "sun-snatching bacteria" are more widespread than previously thought and could change how oceans are affected by global warming. "The findings break from the traditional interpretation of marine ecology found in textbooks, which states that nearly all sunlight in the ocean is captured by chlorophyll in algae. Instead, rhodopsin-equipped bacteria function like hybrid cars, powered by organic matter when available — as most bacteria are — and by sunlight when nutrients are scarce."[33][25]
There is an astrobiological conjecture called the Purple Earth hypothesis which surmises that original life forms on Earth were retinal-based rather than chlorophyll-based, which would have made the Earth appear purple instead of green.[34][35]
Marine algae
Algae is an informal term for a widespread and diverse collection of photosynthetic eukaryotic organisms which are not necessarily closely related and are thus polyphyletic. Unlike higher plants, algae lack roots, stems, or leaves.
Algal groups
Marine algae have traditionally been placed in groups such as: green algae, red algae, brown algae, diatoms, coccolithophores and dinoflagellates.
Green algae
Green algae live most of their lives as single cells or are filamentous, while others form colonies made up from long chains of cells, or are highly differentiated macroscopic seaweeds. They form an informal group containing about 8,000 recognized species.[42]
Red algae
Modern red algae are mostly multicellular with differentiated cells and include many notable seaweeds.[43][44] As coralline algae, they play an important role in the ecology of coral reefs. They form a (disputed) phylum containing about 7,000 recognized species.[43]
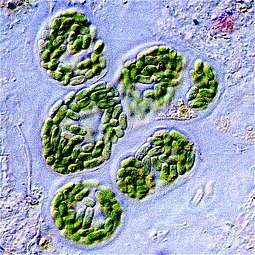
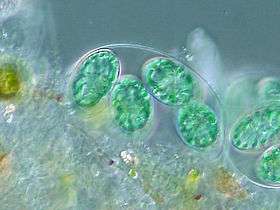
 Cyanidiophyceae colony, a class of unicellular red algae
Cyanidiophyceae colony, a class of unicellular red algae- The seaweed Porphyra umbilicalis
Brown algae
Brown algae are mostly multicellular and include many seaweeds, including kelp. They form a class containing about 2,000 recognized species.[45]
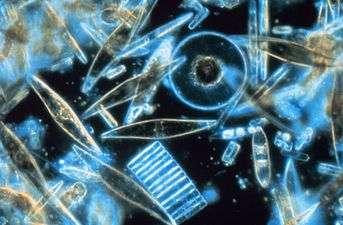
Diatoms
Altogether, about 45 percent of the primary production in the oceans is contributed by diatoms.[46]
Coccolithophores
 The ubiquitous Emiliania huxleyi
The ubiquitous Emiliania huxleyi Emiliania huxleyi bloom off south England
Emiliania huxleyi bloom off south England
Coccolithophores are almost exclusively marine and are found in large numbers throughout the sunlight zone of the ocean. They have calcium carbonate plates (or scales) of uncertain function called coccoliths, which are important microfossils. Coccolithophores are of interest to those studying global climate change because as ocean acidity increases, their coccoliths may become even more important as a carbon sink.[49] The most abundant species of coccolithophore, Emiliania huxleyi is an ubiquitous component of the plankton base in marine food webs.[50] Management strategies are being employed to prevent eutrophication-related coccolithophore blooms, as these blooms lead to a decrease in nutrient flow to lower levels of the ocean.[51]
Dinoflagellate
 Dinoflagellates
Dinoflagellates Karenia brevis produces red tides highly toxic to humans[52]
Karenia brevis produces red tides highly toxic to humans[52]_by_Noctiluca_in_Nagasaki.jpg)
Mixotrophic algae
Other groups
 Diplonemids may be abundant in the world oceans
Diplonemids may be abundant in the world oceans
Traditionally the phylogeny of microorganisms, such as the algal groups discussed above, was inferred and their taxonomy established based on studies of morphology. However developments in molecular phylogenetics have allowed the evolutionary relationship of species to be established by analyzing their DNA and protein sequences.[53] Many taxa, including the algal groups discussed above, are in the process of being reclassified or redefined using molecular phylogenetics. Recent developments in molecular sequencing have allowed for the recovery of genomes directly from environmental samples and avoiding the need for culturing. This has lead for example, to a rapid expansion in knowledge of the abundance and diversity of marine microorganisms. Molecular techniques such as genome-resolved metagenomics and single cell genomics are being used in combination with high throughput techniques.
Between 2009 and 2013, the Tara Oceans expedition traversed the world oceans collecting plankton and analysing them with contemporary molecular techniques. They found a huge range of previously unknown photosynthetic and mixotrophic algae.[54] Among their findings were the diplonemids. These organisms are generally colourless and oblong in shape, typically about 20µm long and with two flagella.[55] Evidence from DNA barcoding suggests diplonemids may be among the most abundant and most species-rich of all marine eukaryote groups.[56][57]
By size
Algae can be classified by size as microalgae or macroalgae.
Microalgae
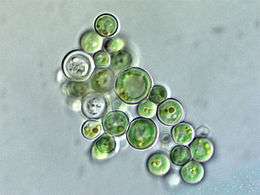
Microalgae are the microscopic types of algae, not visible to the naked eye. They are mostly unicellular species which exist as individuals or in chains or groups, though some are multicellular. Microalgae are important components of the marine protists, as well as the marine phytoplankton. They are very diverse. It has been estimated there are 200,000-800,000 species of which about 50,000 species have been described.[58] Depending on the species, their sizes range from a few micrometers (µm) to a few hundred micrometers. They are specially adapted to an environment dominated by viscous forces.
- Microalgae
 Zooxanthellae is a photosynthetic algae that lives inside hosts like coral
Zooxanthellae is a photosynthetic algae that lives inside hosts like coral
_(cropped).jpg) Euglena mutabilis, a photosynthetic flagellate
Euglena mutabilis, a photosynthetic flagellate
Macroalgae

Macroalgae are the larger, multicellular and more visible types of algae, commonly called seaweeds. Seaweeds usually grow in shallow coastal waters where they are anchored to the seafloor by a holdfast. Seaweed that becomes adrift can wash up on beaches. Kelp is a large brown seaweed that forms large underwater forests covering about 25% of the world coastlines.[59] They are among the most productive and dynamic ecosystems on Earth.[60] Some Sargassum seaweeds are planktonic (free-floating) and form floating drifts.[61](pp246–255) Like microalgae, macroalgae (seaweeds) are technically marine protists since they are not true plants.

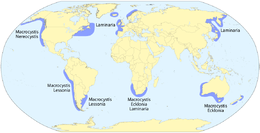
- Macroalgae
 Giant kelp is technically a protist since it is not a true plant, yet it is multicellular and can grow to 50 m
Giant kelp is technically a protist since it is not a true plant, yet it is multicellular and can grow to 50 m- Sargassum seaweed is a brown alga with air bladders that help it float
 Sargassum fish are camouflaged to live among drifting Sargassum seaweed
Sargassum fish are camouflaged to live among drifting Sargassum seaweed
Marine plants
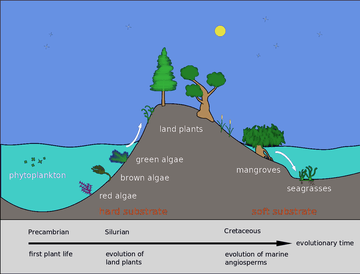
Back in the Silurian, some phytoplankton evolved into red, brown and green algae. These algae then invaded the land and started evolving into the land plants we know today. Later, in the Cretaceous, some of these land plants returned to the sea as mangroves and seagrasses.[63]
Plant life can flourish in the brackish waters of estuaries, where mangroves or cordgrass or beach grass might grow. Flowering plants grow in sandy shallows in the form of seagrass meadows,[64] mangroves line the coast in tropical and subtropical regions[65] and salt-tolerant plants thrive in regularly inundated salt marshes.[66] All of these habitats are able to sequester large quantities of carbon and support a biodiverse range of larger and smaller animal life.[67] Marine plants can be found in intertidal zones and shallow waters, such as seagrasses like eelgrass and turtle grass, Thalassia. These plants have adapted to the high salinity of the ocean environment.
Light is only able to penetrate the top 200 metres (660 ft) so this is the only part of the sea where plants can grow.[68] The surface layers are often deficient in biologically active nitrogen compounds. The marine nitrogen cycle consists of complex microbial transformations which include the fixation of nitrogen, its assimilation, nitrification, anammox and denitrification.[69] Some of these processes take place in deep water so that where there is an upwelling of cold waters, and also near estuaries where land-sourced nutrients are present, plant growth is higher. This means that the most productive areas, rich in plankton and therefore also in fish, are mainly coastal.[70](pp160–163)
Mangroves
Mangroves provide important nursery habitats for marine life, acting as hiding and foraging places for larval and juvenile forms of larger fish and invertebrates. Based on satellite data, the total world area of mangrove forests was estimated in 2010 as 134,257 square kilometres (51,837 sq mi).[71][72]
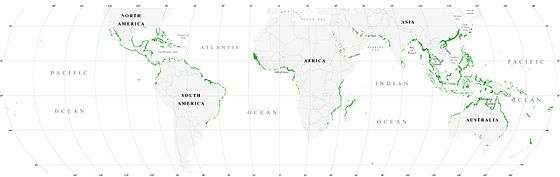
- Spalding, M. (2010) World atlas of mangroves, Routledge. ISBN 9781849776608. doi:10.4324/9781849776608.
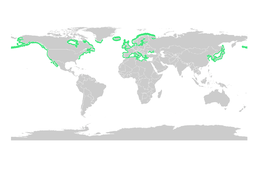
Seagrasses
Like mangroves, seagrasses provide important nursery habitats for larval and juvenile forms of larger fish and invertebrates. The total world area of seagrass meadows is more difficult to determine than mangrove forests, but was conservatively estimated in 2003 as 177,000 square kilometres (68,000 sq mi).[73]

 Sea dragons camouflaged to look like floating seaweed live in kelp forests and seagrass meadows[74]
Sea dragons camouflaged to look like floating seaweed live in kelp forests and seagrass meadows[74]
Evolutionary timeline
.png)
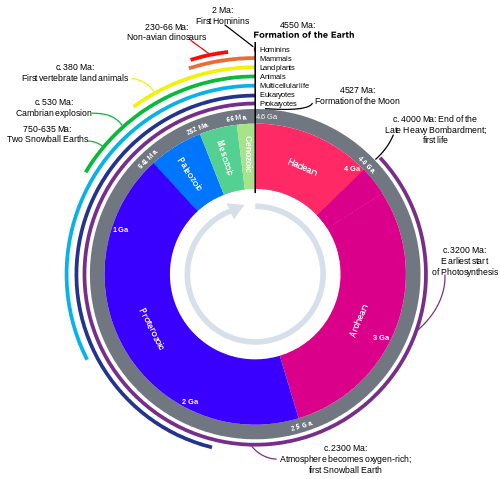
See also
References
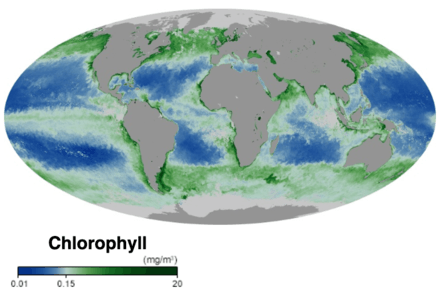
- Chlorophyll NASA Earth Observatory. Accessed 30 November 2019.
- Campbell, Neil A.; Reece, Jane B.; Urry, Lisa Andrea; Cain, Michael L.; Wasserman, Steven Alexander; Minorsky, Peter V.; Jackson, Robert Bradley (2008). Biology (8 ed.). San Francisco: Pearson – Benjamin Cummings. ISBN 978-0-321-54325-7.CS1 maint: ref=harv (link)
- McNeill, J.; et al., eds. (2012). International Code of Nomenclature for algae, fungi, and plants (Melbourne Code), Adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011 (electronic ed.). International Association for Plant Taxonomy. Retrieved 2017-05-14.
- Schäfer G, Engelhard M, Müller V (1 September 1999). "Bioenergetics of the Archaea". Microbiol. Mol. Biol. Rev. 63 (3): 570–620. doi:10.1128/MMBR.63.3.570-620.1999. PMC 103747. PMID 10477309.
- Roach, John (June 7, 2004). "Source of Half Earth's Oxygen Gets Little Credit". National Geographic News. Retrieved 2016-04-04.
- Lin, I.; Liu, W. Timothy; Wu, Chun-Chieh; Wong, George T. F.; Hu, Chuanmin; Chen, Zhiqiang; Wen-Der, Liang; Yang, Yih; Liu, Kon-Kee (2003). "New evidence for enhanced ocean primary production triggered by tropical cyclone". Geophysical Research Letters. 30 (13). doi:10.1029/2003GL017141.
- Sigman, D.M.; Hain, M.P. (2012). "The Biological Productivity of the Ocean" (PDF). Nature Education Knowledge. 3 (6): 1–16. Retrieved 2015-06-01.
The deep chlorophyll maximum (DCM) occurs at the contact where there is adequate light for photosynthesis and yet significant nutrient supply from below.
- Mora, C.; et al. (2013). "Biotic and Human Vulnerability to Projected Changes in Ocean Biogeochemistry over the 21st Century". PLoS Biology. 11 (10): e1001682. doi:10.1371/journal.pbio.1001682. PMC 3797030. PMID 24143135.
- Walsh PJ, Smith S, Fleming L, Solo-Gabriele H, Gerwick WH, eds. (2 September 2011). "Cyanobacteria and cyanobacterial toxins". Oceans and Human Health: Risks and Remedies from the Seas. Academic Press. pp. 271–296. ISBN 978-0-08-087782-2.
- "The Rise of Oxygen - Astrobiology Magazine". Astrobiology Magazine. 30 July 2003. Retrieved 2016-04-06.
- Flannery, D. T.; R.M. Walter (2012). "Archean tufted microbial mats and the Great Oxidation Event: new insights into an ancient problem". Australian Journal of Earth Sciences. 59 (1): 1–11. Bibcode:2012AuJES..59....1F. doi:10.1080/08120099.2011.607849.
- Rothschild, Lynn (September 2003). "Understand the evolutionary mechanisms and environmental limits of life". NASA. Archived from the original on 11 March 2012. Retrieved 13 July 2009.
- Nadis S (December 2003). "The cells that rule the seas" (PDF). Scientific American. 289 (6): 52–3. Bibcode:2003SciAm.289f..52N. doi:10.1038/scientificamerican1203-52. PMID 14631732. Archived from the original (PDF) on 2014-04-19. Retrieved 2019-07-11.
- "The Most Important Microbe You've Never Heard Of". npr.org.
- Flombaum, P.; Gallegos, J. L.; Gordillo, R. A.; Rincon, J.; Zabala, L. L.; Jiao, N.; Karl, D. M.; Li, W. K. W.; Lomas, M. W.; Veneziano, D.; Vera, C. S.; Vrugt, J. A.; Martiny, A. C. (2013). "Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus". Proceedings of the National Academy of Sciences. 110 (24): 9824–9829. Bibcode:2013PNAS..110.9824F. doi:10.1073/pnas.1307701110. PMC 3683724. PMID 23703908.
- Nabors, Murray W. (2004). Introduction to Botany. San Francisco, CA: Pearson Education, Inc. ISBN 978-0-8053-4416-5.
- Allaby, M., ed. (1992). "Algae". The Concise Dictionary of Botany. Oxford: Oxford University Press.
- Grotewold, E. (2006). "The Genetics and Biochemistry of Floral Pigments". Annual Review of Plant Biology. 57: 761–780. doi:10.1146/annurev.arplant.57.032905.105248.
- Lee, DW (2007) Nature's palette - the science of plant color. University of Chicago Press
- Concepts of Biology: Eukaryotic Origins. OpenStax CNX. Retrieved 16 July 2020.

- "chloroplast". Online Etymology Dictionary.
- Basic Biology (18 March 2016). "Bacteria".
- Patrick J. Keeling (2004). "Diversity and evolutionary history of plastids and their hosts". American Journal of Botany. 91 (10): 1481–1493. doi:10.3732/ajb.91.10.1481. PMID 21652304.
- DeLong, E.F.; Beja, O. (2010). "The light-driven proton pump proteorhodopsin enhances bacterial survival during tough times". PLOS Biology. 8 (4): e1000359. doi:10.1371/journal.pbio.1000359. PMC 2860490. PMID 20436957. e1000359.
- Gómez-Consarnau, L.; Raven, J.A.; Levine, N.M.; Cutter, L.S.; Wang, D.; Seegers, B.; Arístegui, J.; Fuhrman, J.A.; Gasol, J.M.; Sañudo-Wilhelmy, S.A. (2019). "Microbial rhodopsins are major contributors to the solar energy captured in the sea". Science Advances. 5 (8): eaaw8855. Bibcode:2019SciA....5.8855G. doi:10.1126/sciadv.aaw8855. PMC 6685716. PMID 31457093.
- Oren, Aharon (2002). "Molecular ecology of extremely halophilic Archaea and Bacteria". FEMS Microbiology Ecology. 39 (1): 1–7. doi:10.1111/j.1574-6941.2002.tb00900.x. ISSN 0168-6496. PMID 19709178.
- Béja, O.; Aravind, L.; Koonin, E.V.; Suzuki, M.T.; Hadd, A.; Nguyen, L.P.; Jovanovich, S.B.; Gates, C.M.; Feldman, R.A.; Spudich, J.L.; Spudich, E.N. (2000). "Bacterial rhodopsin: evidence for a new type of phototrophy in the sea". Science. 289 (5486): 1902–1906. Bibcode:2000Sci...289.1902B. doi:10.1126/science.289.5486.1902. PMID 10988064.
- "Interviews with Fellows: Ed Delong". American Academy of Microbiology. Archived from the original on 7 August 2016. Retrieved 2 July 2016.
- Bacteria with Batteries, Popular Science, January 2001, Page 55.
- Béja, O.; Aravind, L.; Koonin, E.V.; Suzuki, M.T.; Hadd, A.; Nguyen, L.P.; Jovanovich, S.B.; Gates, C.M.; Feldman, R.A.; Spudich, J.L.; Spudich, E.N. (2000). "Bacterial rhodopsin: evidence for a new type of phototrophy in the sea". Science. 289 (5486): 1902–1906. Bibcode:2000Sci...289.1902B. doi:10.1126/science.289.5486.1902. PMID 10988064.
- Boeuf, Dominique; Audic, Stéphane; Brillet-Guéguen, Loraine; Caron, Christophe; Jeanthon, Christian (2015). "MicRhoDE: a curated database for the analysis of microbial rhodopsin diversity and evolution". Database. 2015: bav080. doi:10.1093/database/bav080. ISSN 1758-0463. PMC 4539915. PMID 26286928.
- Yawo, Hiromu; Kandori, Hideki; Koizumi, Amane (5 June 2015). Optogenetics: Light-Sensing Proteins and Their Applications. Springer. pp. 3–4. ISBN 978-4-431-55516-2. Retrieved 30 September 2015.
- A tiny marine microbe could play a big role in climate change University of Southern California, Press Room, 8 August 2019.
- DasSarma, Shiladitya; Schwieterman, Edward W. (11 October 2018). "Early evolution of purple retinal pigments on Earth and implications for exoplanet biosignatures". International Journal of Astrobiology: 1–10. arXiv:1810.05150. Bibcode:2018arXiv181005150D. doi:10.1017/S1473550418000423. ISSN 1473-5504.
- Sparks, William B.; DasSarma, S.; Reid, I. N. (December 2006). "Evolutionary Competition Between Primitive Photosynthetic Systems: Existence of an early purple Earth?". American Astronomical Society Meeting Abstracts. 38: 901. Bibcode:2006AAS...209.0605S.
- Olson, J.M. and Blankenship, R.E. (2005) "Thinking about the evolution of photosynthesis". In: Discoveries in Photosynthesis, pages 1073–1086, Springer. ISBN 9781402033247. doi:10.1007/1-4020-3324-9_95.
- Blankenship, R. E., Sadekar, S., and Raymond, J. (2007) "The evolutionary transition from anoxygenic to oxygenic photosynthesis". In: Evolution of Aquatic Photoautotrophs, eds P. G. Falkowski and A. N. Knoll, New York: Academic Press, pages 21–35. doi:10.1016/B978-012370518-1/50004-7.
- Hohmann-Marriott, M.F. and Blankenship, R.E. (2011) "Evolution of photosynthesis". Annual review of plant biology, 62: 515-548. doi:10.1146/annurev-arplant-042110-103811.
- Kim, E., Harrison, J.W., Sudek, S., Jones, M.D., Wilcox, H.M., Richards, T.A., Worden, A.Z. and Archibald, J.M.(2011) "Newly identified and diverse plastid-bearing branch on the eukaryotic tree of life". Proceedings of the National Academy of Sciences, 108(4): 1496–1500. doi:10.1073/pnas.1013337108.
- Garcia-Mendoza, E. and Ocampo-Alvarez, H. (2011) "Photoprotection in the brown alga Macrocystis pyrifera: evolutionary implications". Journal of Photochemistry and Photobiology B: Biology, 104(1-2): 377–385. doi:10.1016/j.jphotobiol.2011.04.004.
- Shevela, D. (2011) "Adventures with cyanobacteria: a personal perspective". Frontiers in plant science, 2: 28. doi:10.3389/fpls.2011.00028.
- Guiry MD (October 2012). "How many species of algae are there?". Journal of Phycology. 48 (5): 1057–63. doi:10.1111/j.1529-8817.2012.01222.x. PMID 27011267.
- Guiry, M.D.; Guiry, G.M. (2016). "Algaebase". www.algaebase.org. Retrieved November 20, 2016.
- D. Thomas (2002). Seaweeds. Life Series. Natural History Museum, London. ISBN 978-0-565-09175-0.
- Hoek, Christiaan; den Hoeck, Hoeck Van; Mann, David; Jahns, H.M. (1995). Algae : an introduction to phycology. Cambridge University Press. p. 166. ISBN 9780521316873. OCLC 443576944.
- Yool, A.; Tyrrell, T. (2003). "Role of diatoms in regulating the ocean's silicon cycle". Global Biogeochemical Cycles. 17 (4): n/a. Bibcode:2003GBioC..17.1103Y. CiteSeerX 10.1.1.394.3912. doi:10.1029/2002GB002018.
- The Air You're Breathing? A Diatom Made That
- "More on Diatoms". University of California Museum of Paleontology. Archived from the original on 2012-10-04. Retrieved 2019-07-11.
- Smith, H.E.K.; et al. (2012), "Predominance of heavily calcified coccolithophores at low CaCO3 saturation during winter in the Bay of Biscay", Proceedings of the National Academy of Sciences, 109 (23): 8845–8849, Bibcode:2012PNAS..109.8845S, doi:10.1073/pnas.1117508109, PMC 3384182, PMID 22615387
- "Biogeography and dispersal of micro-organisms: a review emphasizing protists", Acta Protozoologica, 45 (2): 111–136, 2005
- Yunev, O.A.; et al. (2007), "Nutrient and phytoplankton trends on the western Black Sea shelf in response to cultural eutrophication and climate changes", Estuarine, Coastal and Shelf Science, 74 (1–2): 63–67, Bibcode:2007ECSS...74...63Y, doi:10.1016/j.ecss.2007.03.030
- Brand, Larry E.; Campbell, Lisa; Bresnan, Eileen (2012). "Karenia: The biology and ecology of a toxic genus". Harmful Algae. 14: 156–178. doi:10.1016/j.hal.2011.10.020.
- Olsen GJ, Woese CR, Overbeek R (1994). "The winds of (evolutionary) change: breathing new life into microbiology". Journal of Bacteriology. 176 (1): 1–6. doi:10.2172/205047. PMC 205007. PMID 8282683.
- Bork, P., Bowler, C., De Vargas, C., Gorsky, G., Karsenti, E. and Wincker, P. (2015) "Tara Oceans studies plankton at planetary scale". doi:10.1126/science.aac5605.
- Gawryluk, Ryan M.R.; Del Campo, Javier; Okamoto, Noriko; Strassert, Jürgen F.H.; Lukeš, Julius; Richards, Thomas A.; Worden, Alexandra Z.; Santoro, Alyson E.; Keeling, Patrick J. (2016). "Morphological Identification and Single-Cell Genomics of Marine Diplonemids". Current Biology. 26 (22): 3053–3059. doi:10.1016/j.cub.2016.09.013. PMID 27875688.
- Faktorová, D., Dobáková, E., Peña-Diaz, P. and Lukeš, J., 2016. From simple to supercomplex: mitochondrial genomes of euglenozoan protists. F1000Research, 5. doi:10.12688/f1000research.8040.1.

- De Vargas, C., Audic, S., Henry, N., Decelle, J., Mahé, F., Logares, R., Lara, E., Berney, C., Le Bescot, N., Probert, I., Carmichael, M. and 44 others (2015) "Eukaryotic plankton diversity in the sunlit ocean. Science", 348(6237): 1261605. doi:10.1126/science.1261605.
- Starckx, Senne (31 October 2012) A place in the sun - Algae is the crop of the future, according to researchers in Geel Flanders Today, Retrieved 8 December 2012
- Kelp Forest - an overview | ScienceDirect Topics
- Mann, K.H. 1973. Seaweeds: their productivity and strategy for growth. Science 182: 975-981.
- Kindersley, Dorling (2011). Illustrated Encyclopedia of the Ocean. Dorling Kindersley. ISBN 978-1-4053-3308-5.
- Tunnell, John Wesley; Chávez, Ernesto A.; Withers, Kim (2007). Coral reefs of the southern Gulf of Mexico. Texas A&M University Press. p. 91. ISBN 978-1-58544-617-9.
- Orth, R.J., Carruthers, T.J., Dennison, W.C., Duarte, C.M., Fourqurean, J.W., Heck, K.L., Hughes, A.R., Kendrick, G.A., Kenworthy, W.J., Olyarnik, S. and Short, F.T. (2006) "A global crisis for seagrass ecosystems". Bioscience, 56(12): pages 987–996. doi:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
- van der Heide, T.; van Nes, E. H.; van Katwijk, M. M.; Olff, H.; Smolders, A. J. P. (2011). Romanuk, Tamara (ed.). "Positive feedbacks in seagrass ecosystems: evidence from large-scale empirical data". PLOS ONE. 6 (1): e16504. Bibcode:2011PLoSO...616504V. doi:10.1371/journal.pone.0016504. PMC 3025983. PMID 21283684.
- "Mangal (Mangrove)". Mildred E. Mathias Botanical Garden. Retrieved 11 July 2013.
- "Coastal Salt Marsh". Mildred E. Mathias Botanical Garden. Retrieved 11 July 2013.
- "Facts and figures on marine biodiversity". Marine biodiversity. UNESCO. 2012. Retrieved 11 July 2013.
- Russell, F. S.; Yonge, C. M. (1928). The Seas. Frederick Warne. pp. 225–227.
- Voss, Maren; Bange, Hermann W.; Dippner, Joachim W.; Middelburg, Jack J.; Montoya, Joseph P.; Ward, Bess (2013). "The marine nitrogen cycle: recent discoveries, uncertainties and the potential relevance of climate change". Philosophical Transactions of the Royal Society B. 368 (1621): 20130121. doi:10.1098/rstb.2013.0121. PMC 3682741. PMID 23713119.
- Stow, Dorrik (2004). Encyclopedia of the Oceans. Oxford University Press. ISBN 978-0-19-860687-1.
- Giri C, Ochieng E, Tieszen LL, Zhu Z, Singh A, Loveland T, et al. (2011) "Status and distribution of mangrove forests of the world using earth observation satellite data". Global Ecology and Biogeography, 20(1):154–159. doi:10.1111/j.1466-8238.2010.00584.x
- Thomas, N., Lucas, R., Bunting, P., Hardy, A., Rosenqvist, A. and Simard, M. (2017) "Distribution and drivers of global mangrove forest change, 1996–2010". PLOS ONE, 12(6): e0179302. doi:10.1371/journal.pone.0179302
- Short, F.T. and Frederick, T. (2003) World atlas of seagrasses, University of California Press, page 24. ISBN 9780520240476
- Froese, Rainer and Pauly, Daniel, eds. (2009). "Phycodurus eques" in FishBase. July 2009 version.
- Hassani, M.A., Durán, P. and Hacquard, S. (2018) "Microbial interactions within the plant holobiont". Microbiome, 6(1): 58. doi:10.1186/s40168-018-0445-0.

- Lucking, R., Huhndorf, S., Pfister, D.H., Plata, E.R. and Lumbsch, H.T. (2009) "Fungi evolved right on track". Mycologia, 101(6): 810–822. doi:10.3852/09-016.
- Heckman, D.S., Geiser, D.M., Eidell, B.R., Stauffer, R.L., Kardos, N.L. and Hedges, S.B. (2001) "Molecular evidence for the early colonization of land by fungi and plants". Science, 293(5532): 1129–1133. doi:10.1126/science.1061457.
Further reading
- Falkowski, Paul (Ed.) (2013) Primary Productivity in the Sea Springer. ISBN 9781468438901.
- Falkowski, Paul and Raven, John A. (2013) Aquatic Photosynthesis Second edition revised, Princeton University Press. ISBN 9781400849727.
- Falkowski P and Knoll AH (2011) Evolution of Primary Producers in the Sea Academic Press. ISBN 9780080550510.
- Kirk, John T. O. (2010) Light and Photosynthesis in Aquatic Ecosystems Third edition revised, Cambridge University Press. ISBN 9781139493918.
.jpg)
_Various_diatoms.jpg)