Anammox
Anammox, an abbreviation for anaerobic ammonium oxidation, is a globally important microbial process of the nitrogen cycle[1] that takes place in many natural environments. The bacteria mediating this process were identified in 1999, and were a great surprise for the scientific community.[2] In the anammox reaction, nitrite and ammonium ions are converted directly into Diatomic Nitrogen and water.

The bacteria that perform the anammox process are genera that belong to the bacterial phylum Planctomycetes. The anammox bacteria all possess one anammoxosome, a lipid bilayer membrane-bound compartment inside the cytoplasm in which the anammox process takes place. The anammoxosome membranes are rich in ladderane lipids; the presence of these lipids is so far unique in biology.
"Anammox" is also the trademarked name for an anammox-based ammonium removal technology developed[3] by the Delft University of Technology.
Process background
In this biological process, which is a comproportionation reaction, nitrite and ammonium ions are converted directly into diatomic nitrogen and water.[5]
- NH4+ + NO2− → N2 + 2H2O.
Globally, this process may be responsible for 30-50% of the N2 gas produced in the oceans.[6] It is thus a major sink for fixed nitrogen and so limits oceanic primary productivity.
The bacteria that perform the anammox process belong to the bacterial phylum Planctomycetes. Currently, five anammox genera have been discovered: Brocadia, Kuenenia, Anammoxoglobus, Jettenia (all fresh water species), and Scalindua (marine species).[7] The anammox bacteria are characterized by several striking properties: they all possess one anammoxosome, a membrane bound compartment inside the cytoplasm which is the locus of anammox catabolism. Further, the membranes of these bacteria mainly consist of ladderane lipids so far unique in biology.[8] Of special interest is the conversion to hydrazine (normally used as a high-energy rocket fuel, and poisonous to most living organisms) as an intermediate.[9] A final striking feature of the organism is the extremely slow growth rate. The doubling time is anywhere from 7–22 days.[4] The anammox bacteria are geared towards converting their substrates at very low concentrations; in other words, they have a very high affinity to their substrates ammonium and nitrite (sub-micromolar range).[10][11] Anammox cells are packed with cytochrome c type proteins (≈30% of the protein complement), including the enzymes that perform the key catabolic reactions of the anammox process, making the cells remarkably red.[12] The anammox process was originally found to occur only from 20 °C to 43 °C[10] but more recently, anammox has been observed at temperatures from 36 °C to 52 °C in hot springs[13] and 60 °C to 85 °C at hydrothermal vents located along the Mid-Atlantic Ridge.[14]
History
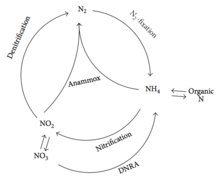
In 1932, it was reported that dinitrogen gas was generated via an unknown mechanism during fermentation in the sediments of Lake Mendota, Wisconsin, USA.[15] In 1965, F. A. Richards[16] noticed that most of the ammonium that should be produced during the anaerobic remineralization of organic matter was unaccounted for. As there was no known biological pathway for this transformation, biological anaerobic oxidation of ammonium received little further attention.[17] In 1977, Engelbert Broda predicted the existence of two chemolithoautotrophic microorganisms capable of oxidizing ammonium to dinitrogen gas on the basis of thermodynamic calculations.[18] It was thought that anaerobic oxidation of ammonium would not be feasible, assuming that the predecessors had tried and failed to establish a biological basis for those reactions. By the 1990s, Arnold Mulder's observations were just consistent with Richard's suggestion.[19] In their anoxic denitrifying pilot reactor, ammonium disappeared at the expense of nitrite with a clear nitrogen production. The reactor used the effluent from a methanogenic pilot reactor, which contained ammonium, sulphide and other compounds, and nitrate from a nitrifying plant as the influent. The process was named "anammox," and was realized to have great significance in the removal of unwanted ammonium. The discovery of the anammox process was first publicly presented at the 5th European congress on biotechnology.[20] By the mid-1990s, the discovery of anammox in the fluidized bed reactor was published.[21] A maximum ammonium removal rate of 0.4 kg N/m3/d was achieved. It was shown that for every mole of ammonium consumed, 0.6 mol of nitrate was required, resulting in the formation of 0.8 mol of N2 gas. In the same year, the biological nature of anammox was identified.[22] Labeling experiments with 15NH4+ in combination with 14NO3− showed that 14-15N2 was the dominant product making up 98.2% of the total labeled N2. It was realized that, instead of nitrate, nitrite was assumed as the oxidizing agent of ammonium in anammox reaction. Based on a previous study, Strous et al.[23] calculated the stoichiometry of anammox process by mass balancing, which is widely accepted by other groups. Later, anammox bacteria were identified as planctomycetes,[24] and the first identified anammox organism was named Candidatus "Brocadia anammoxidans."[25] Before 2002, anammox was assumed to be a minor player in the nitrogen cycle within natural ecosystems.[26] In 2002 however, anammox was found to play an important part in the biological nitrogen cycle, accounting for 24-67% of the total N2 production in the continental shelf sediments that were studied.[27][28] The discovery of anammox process modified the concept of biological nitrogen cycle, as depicted in Figure 2.
Possible reaction mechanisms
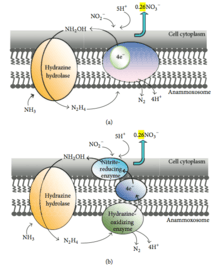
According to 15N labeling experiments carried out in 1997, ammonium is biologically oxidized by hydroxylamine, most likely derived from nitrite, as the probable electron acceptor.[29] The conversion of hydrazine to dinitrogen gas is hypothesized to be the reaction that generates the electron equivalents for the reduction of nitrite to hydroxylamine.[30] In general, two possible reaction mechanisms are addressed.[31] One mechanism hypothesizes that a membrane-bound enzyme complex converts ammonium and hydroxylamine to hydrazine first, followed by the oxidation of hydrazine to dinitrogen gas in the periplasm. At the same time, nitrite is reduced to hydroxylamine at the cytoplasmic site of the same enzyme complex responsible for hydrazine oxidation with an internal electron transport (Figure 3a). The other mechanism postulates the following: ammonium and hydroxylamine are converted to hydrazine by a membrane-bound enzyme complex, hydrazine is oxidized in the periplasm to dinitrogen gas, and the generated electrons are transferred via an electron transport chain to nitrite reducing enzyme in the cytoplasm where nitrite is reduced to hydroxylamine (Figure 3b). Whether the reduction of nitrite and the oxidation of hydrazine occur at different sites of the same enzyme or the reactions are catalyzed by different enzyme systems connected via an electron transport chain remains to be investigated.[30] In microbial nitrogen metabolism, the occurrence of hydrazine as an intermediate is rare.[32] Hydrazine has been proposed as an enzyme-bound intermediate in the nitrogenase reaction.[33] Recently, using detailed molecular analyses and combining complementary methods, Kartal and coworkers published strong evidence supporting the latter mechanism.[12][34] Furthermore, the enzyme producing hydrazine, hydrazine synthase was purified and shown to produce hydrazine from NO and ammonium.[12] The production of hydrazine from ammonium and NO was also supported by the resolution of the crystal structure of the enzyme hydrazine sythase.[35]
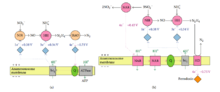
A possible role of nitric oxide (NO) or nitroxyl (HNO) in anammox was proposed by Hooper et al.[36] by way of condensation of NO or HNO and ammonium on an enzyme related to the ammonium monooxygenase family. The formed hydrazine or imine could subsequently be converted by the enzyme hydroxylamine oxidase to dinitrogen gas, and the reducing equivalents produced in the reaction are required to combine NO or HNO and ammonium or to reduce nitrite to NO. Environmental genomics analysis of the species Candidatus Kuenenia stuttgartiensis, through a slightly different and complementary metabolism mechanism, suggested NO to be the intermediate instead of hydroxylamine (Figure 4).[37] However, this hypothesis also agreed that hydrazine was an important intermediate in the process. In this pathway (Figure 4), there are two enzymes unique to anammox bacteria: hydrazine synthase (hzs) and hydrazine dehydrogenase (hdh). The HZS produces hydrazine from nitric oxide and ammonium, and HDH transfer the electrons from hydrazine to ferredoxin. Few new genes, such as some known fatty acid biosynthesis and S-adenosylmethionine radical enzyme genes, containing domains involved in electron transfer and catalysis have been detected.[37] Anammox microorganisms can also directly couple NO reduction to ammonia oxidation, without the need for nitrite supply.[38]
Another, still unexplored, reaction mechanism involves anaerobic ammonium oxidation on anodes of bio-electrical systems. Such systems can be microbial fuel cells or microbial electrolysis cells. In the absence of dissolved oxygen, nitrite, or nitrate, microbes living in the anode compartment are able to oxidize ammonium to dinitrogen gas (N2) just as in the classical anammox process.[39] At the same time, they unload the liberated electrons onto the anode, producing electrical current. This electrical current can be used either directly in fuel cell mode[40] or for hydrogen and methane gas production in electrolysis mode.[39] While there is no clarity on the reaction mechanism behind, one hypothesis is that nitrite, nitrate, or dinitrogen oxide play a role as intermediates.[40] However, since the process occurs at very low electrochemical potentials, other, more speculative, reaction mechanisms seem possible as well.
Species diversity
Until now, ten anammox species have been described, including seven that are available in laboratory enrichment cultures.[4] All have the taxonomical status of Candidatus, as none were obtained as classical pure cultures. Known species are divided over five genera: (1) Kuenenia, represented by Kuenenia stuttgartiensis,[37] (2) Brocadia (three species: B. anammoxidans, B. fulgida, and B. sinica),[24][41][42] (3) Anammoxoglobus (one species: A. propionicus,[43] (4) Jettenia (one species: J. asiatica,[44][45] and (5) Scalindua (four species: S. brodae, S. sorokinii, S. wagneri, and S. profunda[46][47][48] Representatives of the first four genera were enriched from sludge from wastewater treatment plants; K. stuttgartiensis, B. anammoxidans, B. fulgida, and A. propionicus were even obtained from the same inoculum. Scalindua dominates the marine environment, but is also found in some freshwater ecosystems and wastewater treatment plants.[46][49][50][51] Together, these 10 species likely only represent a minute fraction of anammox biodiversity. For instance, there are currently over 2000 16S rRNA gene sequences affiliated with anammox bacteria that have been deposited to the Genbank (https://www.ncbi.nlm.nih.gov/genbank/), representing an overlooked continuum of species, subspecies, and strains, each apparently having found its specific niche in the wide variety of habitats where anammox bacteria are encountered. Species microdiversity is particularly impressive for the marine representative Scalindua.[47][52][53][54][55][56] A question that remains to be investigated is which environmental factors determine species differentiation among anammox bacteria.
The sequence identities of the anammox 16S rRNA genes range from 87 to 99%, and phylogenetic analysis places them all within the phylum Planctomycetes,[57] which form the PVC superphylum together with Verrucomicrobia and Chlamydiae.[58] Within the Planctomycetes, anammox bacteria deeply branch as a monophyletic clade. Their phylogenetic position together with a broad range of specific physiological, cellular, and molecular traits give anammox bacteria their own order Brocadiales.[59]
Application
The application of the anammox process lies in the removal of ammonium in wastewater treatment and consists of two separate processes. The first step is partial nitrification (nitritation) of half of the ammonium to nitrite by ammonia oxidizing bacteria:
- 2NH4+ + 3O2 → 2NO2− + 4H+ + 2H2O
The resulting ammonium and nitrite are converted in the anammox process to dinitrogen gas and circa 15% nitrate (not shown) by anammox bacteria:
- NH4+ + NO2− → N2 + 2 H2O
Both processes can take place in 1 reactor where two guilds of bacteria form compact granules.[60][61]
For the enrichment of the anammox organisms a granular biomass or biofilm system seems to be especially suited in which the necessary sludge age of more than 20 days can be ensured. Possible reactors are sequencing batch reactors (SBR), moving bed reactors or gas-lift-loop reactors. The cost reduction compared to conventional nitrogen removal is considerable; the technique is still young but proven in several fullscale installations. The first full scale reactor intended for the application of anammox bacteria was built in the Netherlands in 2002.[62] In other wastewater treatment plants, such as the one in Germany (Hattingen), anammox activity is coincidentally observed though were not built for that purpose. As of 2006, there are three full scale processes in The Netherlands: one in a municipal wastewater treatment plant (in Rotterdam), and two on industrial effluent. One is a tannery, the other a potato processing plant.
Advantages
Conventional nitrogen removal from ammonium-rich wastewater is accomplished in two separate steps: nitrification, which is mediated by aerobic ammonia- and nitrite-oxidizing bacteria and denitrification carried out by denitrifiers, which reduce nitrate to N2 with the input of suitable electron donors. Aeration and input of organic substrates (typically methanol) show that these two processes are: (1) highly energy consuming, (2) associated with the production of excess sludge and (3) produce significant amounts of green-house gases such as CO2 and N2O and ozone-depleting NO.[63] Because anammox bacteria convert ammonium and nitrite directly to N2 anaerobically, this process does not require aeration and other electron donors. Nevertheless, oxygen is still required for the production of nitrite by ammonia-oxiding bacteria. However, in partial nitritation/anammox systems, oxygen demand is greatly reduced because only half of the ammonium needs to be oxidized to nitrite instead of full conversion to nitrate. The autotrophic nature of anammox bacteria and ammonia-oxidizing bacteria guarantee a low yield and thus less sludge production.[63] Additionally, anammox bacteria easily form stable self-aggregated biofilm (granules) allowing reliable operation of compact systems characterized by high biomass concentration and conversion rate up to 5–10 kg N m−3.[64] Overall, it has been shown that efficient application of the anammox process in wastewater treatment results in a cost reduction of up to 60%[65][66] as well as lower CO2 emissions.[63]
Disadvantages
The doubling time is slow, between 10 days to 2 weeks.[67] This makes it difficult to grow enough sludge for a wastewater treatment reactor. Also the recovery time after the loss of sludge by accident is longer than in conventional nitrogen removal systems. On the other hand, this slow growing rate is an advantage due to the reduction of surplus sludge that needs to be removed and treated. Depending on the exact species, the optimum pH level is 8.[67] Therefore, it can be necessary to adjust the pH of the wastewater by adding caustic.
References
- Arrigo KR (2005). "Marine microorganisms and global nutrient cycles". Nature. 437 (7057): 349–55. Bibcode:2005Natur.437..349A. doi:10.1038/nature04159. PMID 16163345. S2CID 62781480.
- Strous, M. et al. . "Missing lithotroph identified as new planctomycete". Nature 400(6743): 446–449 (1999).
- Jetten Michael Silvester Maria, Van Loosdrecht Marinus Corneli; Technische Universiteit Delft, patent WO9807664
- Kartal B.; et al. (2013). "How to make a living from anaerobic ammonium oxidation" (PDF). FEMS Microbiology Reviews. 37 (3): 428–461. doi:10.1111/1574-6976.12014. PMID 23210799.
- Reimann, Joachim; Jetten, Mike S.M.; Keltjens, Jan T. (2015). "Chapter 7 Metal Enzymes in "Impossible" Microorganisms Catalyzing the Anaerobic Oxidation of Ammonium and Methane". In Peter M.H. Kroneck and Martha E. Sosa Torres (ed.). Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. 15. Springer. pp. 257–313. doi:10.1007/978-3-319-12415-5_7. PMID 25707470.
- Devol A. H.; et al. (2003). "Nitrogen cycle: solution to a marine mystery". Nature. 422 (6932): 575–576. Bibcode:2003Natur.422..575D. doi:10.1038/422575a. PMID 12686985. S2CID 7789698.
- Jetten, M. S. M. et al. Biochemistry and molecular biology of anammox bacteria" Critical Reviews in Biochemistry and Molecular Biology 44(2-3), 65-84 (2009)
- Boumann H. A.; et al. (2009). "Biophysical properties of membrane lipids of anammox bacteria: I. Ladderane phospholipids form highly organized fluid membranes". Biochim Biophys Acta. 1788 (7): 1444–1451. doi:10.1016/j.bbamem.2009.04.008. PMID 19376084.
- "Pee power: Urine-loving bug churns out space fuel". Agence France Press. 2011-10-02. Retrieved 2011-10-03.
- Strous, M., Kuenen, J.G., Jetten, M.S. 1999. Key Physiology of Anaerobic Ammonium Oxidation. App. Environ. Microb. (3248-3250)
- Yan, J; Haaijer, SCM; Op den Camp, HJM; van Niftrik, L; Stahl, DA; Konneke, M; Rush, D; Sinninghe Damste, JS; Hu, YY; Jetten, MSM (September 2012). "Mimicking the oxygen minimum zones: stimulating interaction of aerobic archaeal and anaerobic bacterial ammonia oxidizers in a laboratory-scale model system". Environ Microbiol. 14 (12): 3146–3158. doi:10.1111/j.1462-2920.2012.02894.x. PMC 3558802. PMID 23057688.
- Kartal, B; Maalcke, WJ; de Almeida, NM; Cirpus, I; Gloerich, J; Geerts, W; Op den Camp, HJ; Harhangi, HR; Janssen-Megens, EM; Francoijs, KJ; Stunnenberg, HG; Keltjens, JT; Jetten, MS; Strous, M. (2011). "Molecular mechanism of anaerobic ammonium oxidation". Nature. 479 (7371): 127–130. Bibcode:2011Natur.479..127K. doi:10.1038/nature10453. PMID 21964329.
- Jaeschke; et al. (March 2009). "16S rRNA gene and lipid biomarker evidence for anaerobic ammonium-oxidizing bacteria (anammox) in California and Nevada hot springs". FEMS Microbiol. Ecol. 67 (3): 343–350. doi:10.1111/j.1574-6941.2008.00640.x. PMID 19220858.
- Byrne, Nathalie; Strous, Marc; Crepeau, Valentin; et al. (January 2009). "Presence and activity of anaerobic ammonium-oxidizing bacteria at deep-sea hydrothermal vents". The ISME Journal. 3 (1): 117–123. doi:10.1038/ismej.2008.72. PMID 18670398.
- Allgeier, R. J. et al. The anaerobic fermentation of lake deposits. International Review of Hydrobiology 26(5-6), 444-461 (1932)
- F.A. Richards (1965). "Anoxic basins and fjordsin". In J.P. Ripley; G. Skirrow (eds.). Chemical Oceanography. London: Academic Press. pp. 611–645.
- Arrigo, K. R. (2005). "Marine microorganisms and global nutrient cycles". Nature. 437 (7057): 349–355. Bibcode:2005Natur.437..349A. doi:10.1038/nature04159. PMID 16163345. S2CID 62781480.
- Broda, E. (1977). "Two kinds of lithotrophs missing in nature". Zeitschrift für Allgemeine Mikrobiologie. 17 (6): 491–493. doi:10.1002/jobm.3630170611. PMID 930125.
- Kuenen, J. G. (2008). "Anammox bacteria: from discovery to application". Nature Reviews Microbiology. 6 (4): 320–326. doi:10.1038/nrmicro1857. PMID 18340342. S2CID 6378856.
- A. A. van de Graaf, A. Mulder, H. Slijkhuis, L. A. Robertson, and J. G. Kuenen, "Anoxic ammonium oxidation," in Proceedings of the 5th European Congress on Biotechnology, C. Christiansen, L. Munck, and J. Villadsen, Eds., pp. 338–391, Copenhagen, Denmark, 1990
- A. Mulder, A. A. Van De Graaf, L. A. Robertson, and J. G. Kuenen, "Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor," FEMS Microbiology Ecology, vol. 16, no. 3, pp. 177–184, 1995
- Van de Graaf AA, Mulder A, De Bruijn P, Jetten MSM, Robertson LA, Kuenen JG (1995). "Anaerobic oxidation of ammonium is a biologically mediated process". Applied and Environmental Microbiology. 61 (4): 1246–1251. doi:10.1128/AEM.61.4.1246-1251.1995. PMC 167380. PMID 7747947.
- M. Strous, J. J. Heijnen, J. G. Kuenen, and M. S. M. Jetten, "The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms," Applied Microbiology and Biotechnology, vol. 50, no. 5, pp. 589–596, 1998
- M. Strous, J. A. Fuerst, E. H. M. Kramer et al., "Missing lithotroph identified as new planctomycete," Nature, vol. 400, no. 6743, pp. 446–449, 1999
- J. G. Kuenen and M. S. M. Jetten, 2001 "Extraordinary anaerobic ammonium oxidising bacteria," ASM News, vol. 67, pp. 456–463,
- Francis CA, Beman JM, Kuypers MMM) (2007). "New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation". ISME Journal. 1 (1): 19–27. doi:10.1038/ismej.2007.8. PMID 18043610.
- Kuypers, MMM; Marchant, HK; Kartal, B (2011). "The Microbial Nitrogen-Cycling Network". Nature Reviews Microbiology. 1 (1): 1–14. doi:10.1038/nrmicro.2018.9. PMID 29398704. S2CID 3948918.
- Thamdrup B, Dalsgaard T (2002). "Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments". Applied and Environmental Microbiology. 68 (3): 1312–1318. doi:10.1128/aem.68.3.1312-1318.2002. PMC 123779. PMID 11872482.
- Van De Graaf A. A.; et al. (1997). "Metabolic pathway of anaerobic ammonium oxidation on the basis of 15N studies in a fluidized bed reactor". Microbiology. 143 (7): 2415–2421. doi:10.1099/00221287-143-7-2415.
- Ni, S-Q. and Zhang, J. Anaerobic Ammonium Oxidation: From Laboratory to Full-Scale Application" BioMed Research International 2013; 2013, 1-10
- Jetten M. S. M.; et al. (1998). "The anaerobic oxidation of ammonium". FEMS Microbiology Reviews. 22 (5): 421–437. doi:10.1016/s0168-6445(98)00023-0.
- Schalk H.; et al. (1998). ""The anaerobic oxidation of hydrazine " a novel reaction in microbial nitrogen metabolism". FEMS Microbiology Letters. 158 (1): 61–67. doi:10.1016/s0378-1097(97)00501-6. PMID 9453157.
- Dilworth M. J., Eady (1991). "Azotobacter chroococcum". Biochemical Journal. 277 (2): 465–468. doi:10.1042/bj2770465. PMC 1151257. PMID 1859374.
- Kartal B, de Almeida NM, Maalcke WJ, Op den Camp HJ, Jetten MS, Keltjens JT (2013). "How to make a living from anaerobic ammonium oxidation". FEMS Microbiol Rev. 37 (3): 428–461. doi:10.1111/1574-6976.12014. PMID 23210799.
- Dietl A, Ferousi C, Maalcke WJ, Menzel A, de Vries S, Keltjens JT, Jetten MS, Kartal B, Barends TR (Nov 2015). "The inner workings of the hydrazine synthase multiprotein complex". Nature. 527 (7578): 394–7. Bibcode:2015Natur.527..394D. doi:10.1038/nature15517. PMID 26479033. S2CID 205245898.
- Hooper A. B.; et al. (1997). "Enzymology of the oxidation of ammonia to nitrite by bacteria". Antonie van Leeuwenhoek. 71 (1–2): 59–67. doi:10.1023/a:1000133919203. PMID 9049018.
- Strous M.; et al. (2006). "Deciphering the evolution and metabolism of an anammox bacterium from a community genome". Nature. 440 (7085): 790–794. Bibcode:2006Natur.440..790S. doi:10.1038/nature04647. PMID 16598256. S2CID 4402553.
- Hu Z, Wessels HJ, van Alen TA, Jetten MS, Kartal B (March 2019). "Nitric oxide-dependent anaerobic ammonium oxidation". Nature Communications. 10 (1): 1244. Bibcode:2019NatCo..10.1244H. doi:10.1038/s41467-019-09268-w. PMC 6423088. PMID 30886150.
- Siegert, M.; Tan, A. (2019). "Electric stimulation of ammonotrophic methanogenesis". Frontiers in Energy Research. 7: 17. doi:10.3389/fenrg.2019.00017.
- Vilajeliu-Pons, A.; Koch, C.; Balaguer, M.D.; Colprim, J.; Harnisch, F.; Puig, S (2018). "Microbial electricity driven anoxic ammonium removal". Water Research. 130: 168–175. doi:10.1016/j.watres.2017.11.059. PMID 29220717.
- Kartal B.; et al. (2008). "Candidatus 'Brocadia fulgida': an autofluorescent anaerobic ammonium oxidizing bacterium". FEMS Microbiol. Ecol. 63 (1): 46–55. doi:10.1111/j.1574-6941.2007.00408.x. PMID 18081590.
- Oshiki M.; et al. (2011). "Physiological characteristics of the anaerobic ammonium-oxidizing bacterium Candidatus 'Brocadia sinica'". Microbiology. 157 (6): 1706–1713. doi:10.1099/mic.0.048595-0. PMID 21474538.
- Kartal B.; et al. (2007). "Candidatus "Anammoxoglobus propionicus" a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria". Syst Appl Microbiol. 30 (1): 39–49. doi:10.1016/j.syapm.2006.03.004. PMID 16644170.
- Quan Z. X.; et al. (2008). "Diversity of ammonium-oxidizing bacteria in a granular sludge anaerobic ammonium-oxidizing (anammox) reactor". Environ Microbiol. 10 (11): 3130–3139. doi:10.1111/j.1462-2920.2008.01642.x. PMID 18479446.
- Hu B. L.; et al. (2011). "New anaerobic, ammonium-oxidizing community enriched from peat soil". Appl Environ Microbiol. 77 (3): 966–971. doi:10.1128/aem.02402-10. PMC 3028707. PMID 21148690.
- Schmid M.; et al. (2003). "Candidatus "Scalindua brodae", sp. nov., Candidatus "Scalindua wagneri", sp. nov., two new species of anaerobic ammonium oxidizing bacteria". Syst Appl Microbiol. 26 (4): 529–538. doi:10.1078/072320203770865837. PMID 14666981.
- Woebken D.; et al. (2008). "A microdiversity study of anammox bacteria reveals a novel Candidatus Scalindua phylotype in marine oxygen minimum zones". Environ Microbiol. 10 (11): 3106–3119. doi:10.1111/j.1462-2920.2008.01640.x. PMID 18510553.
- Van de Vossenberg J; et al. (2012). "The metagenome of the marine anammox bacterium Candidatus Scalindua profunda' illustrates the versatility of this globally important nitrogen cycle bacterium". Environ Microbiol. 15 (5): 1275–1289. doi:10.1111/j.1462-2920.2012.02774.x. PMC 3655542. PMID 22568606.
- Schubert C. J.; et al. (2006). "Anaerobic ammonium oxidation in a tropical freshwater system (Lake Tanganyika)". Environ Microbiol. 8 (10): 1857–1863. doi:10.1111/j.1462-2920.2006.01074.x. PMID 16958766.
- Hamersley M. R.; et al. (2009). "Water column anammox and denitrification in a temperate permanently stratified lake (Lake Rassnitzer, Germany)". Syst Appl Microbiol. 32 (8): 571–582. doi:10.1016/j.syapm.2009.07.009. PMID 19716251.
- Ligi T.; et al. (2015). "The genetic potential of N2 emission via denitrification and ANAMMOX from the soils and sediments of a created riverine treatment wetland complex". Ecol Eng. 80: 181–190. doi:10.1016/j.ecoleng.2014.09.072.
- Schmid, M. C. et al. Anaerobic ammonium-oxidizing bacteria in marine environments: widespread occurrence but low diversity (2007)
- Dang H.; et al. (2010). "Environmental factors shape sediment anammox bacterial communities in hypernutrified Jiaozhou Bay, China". Appl Environ Microbiol. 76 (21): 7036–7047. doi:10.1128/aem.01264-10. PMC 2976235. PMID 20833786.
- Hong Y. G.; et al. (2011a). "Residence of habitat-specific anammox bacteria in the deep-sea subsurface sediments of the South China Sea: analyses of marker gene abundance with physical chemical parameters". Microb Ecol. 62 (1): 36–47. doi:10.1007/s00248-011-9849-0. PMC 3141849. PMID 21491114.
- Hong Y. G.; et al. (2011b). "Diversity and abundance of anammox bacterial community in the deep-ocean surface sediment from equatorial Pacific". Appl Microbiol Biotechnol. 89 (4): 1233–1241. doi:10.1007/s00253-010-2925-4. PMID 20949269. S2CID 20118397.
- Li M.; et al. (2011). "Spatial distribution and abundances of ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) in mangrove sediments". Appl Microbiol Biotechnol. 89 (4): 1243–1254. doi:10.1007/s00253-010-2929-0. PMC 3035804. PMID 20953601.
- Fuerst J. A., Sagulenko E. (2011). "Beyond the bacterium: planctomycetes challenge our concepts of microbial structure and function". Nat Rev Microbiol. 9 (6): 403–413. doi:10.1038/nrmicro2578. PMID 21572457. S2CID 12498825.
- Wagner M, Horn M (2006). "The Planctomycetes, Verrucomicrobia, Chlamydiae and sister phyla comprise a superphylum with biotechnological and medical relevance". Curr Opin Biotechnol. 17 (3): 241–249. doi:10.1016/j.copbio.2006.05.005. PMID 16704931.
- Jetten MSM, Op den Camp HJM, Kuenen JG & Strous M (2010) Description of the order Brocadiales. Bergey’s Manual of Systematic Bacteriology, Vol 4 (Krieg NR, Ludwig W, Whitman WB, Hedlund BP, Paster BJ, Staley JT, Ward N, Brown D & Parte A, eds), pp. 596–603. Springer, Heidelberg
- Kartal B, Kuenen JG, van Loosdrecht MC (2010). "Sewage treatment with anammox". Science. 328 (5979): 702–703. Bibcode:2010Sci...328..702K. doi:10.1126/science.1185941. PMID 20448175.
- Knight, Helen (May 7, 2010). "Bugs will give us free power while cleaning our sewage". New Scientist. Retrieved 9 May 2010.
- Van der Star WR, Abma WR, Blommers D, Mulder JW, Tokutomi T, Strous M, Picioreanu C, Van Loosdrecht MC (2007). "Startup of reactors for anoxic ammonium oxidation: experiences from the first full-scale anammox reactor in Rotterdam". Water Res. 41 (18): 4149–4163. doi:10.1016/j.watres.2007.03.044. PMID 17583763.
- Hu Z, Lotti T, Lotti T, de Kreuk M, Kleerebezem R, van Loosdrecht M, Kruit J, Jetten MS, Kartal B (2013). "Nitrogen removal by a nitritation-anammox bioreactor at low temperature". Appl Environ Microbiol. 79 (8): 2807–2812. doi:10.1128/AEM.03987-12. PMC 3623191. PMID 23417008.
- van Loosdrecht MCM (2008) Innovative nitrogen removal. In: Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (eds) Biological wastewater treatment: principles, modelling and design. IWA Publishing, London, pp 139–155
- Siegrist H, Salzgeber D, Eugster J, Joss A (2008). "Anammox brings WWTP closer to energy autarky due to increased biogas production and reduced aeration energy for N-removal". Water Sci Technol. 57 (3): 383–388. doi:10.2166/wst.2008.048. PMID 18309216.
- Van Dongen U, Jetten MS, van Loosdrecht MC (2001). "The SHARON((R))-Anammox((R)) process for treatment of ammonium rich wastewater". Water Sci Technol. 44: 153–160. doi:10.2166/wst.2001.0037. S2CID 13354123.
- microbewiki: Anammox