Haloarchaea
Haloarchaea (halophilic archaea, halophilic archaebacteria, halobacteria)[1] are a class of the Euryarchaeota,[2] found in water saturated or nearly saturated with salt. Halobacteria are now recognized as archaea, rather than bacteria and are one of the largest groups. The name 'halobacteria' was assigned to this group of organisms before the existence of the domain Archaea was realized, and while valid according to taxonomic rules, should be updated.[3] Halophilic archaea are generally referred to as haloarchaea to distinguish them from halophilic bacteria.
| Haloarchaea | |
|---|---|
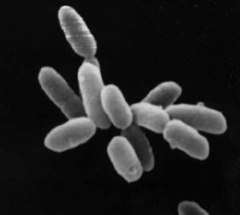 | |
| Halobacterium sp. strain NRC-1, each cell about 5 µm in length. | |
| Scientific classification | |
| Domain: | |
| Kingdom: | |
| Phylum: | |
| Class: | Halobacteria Grant et al. 2002 |
| Order | |
| Synonyms | |
| |
These microorganisms are members of the halophile community, in that they require high salt concentrations to grow, with most species requiring more than 2.0M NaCl for growth and survival.[4] They are a distinct evolutionary branch of the Archaea distinguished by the possession of ether-linked lipids and the absence of murein in their cell walls.
Haloarchaea can grow aerobically or anaerobically. Parts of the membranes of haloarchaea are purplish in color,[5] and large blooms of haloarchaea appear reddish, from the pigment bacteriorhodopsin, related to the retinal pigment rhodopsin, which it uses to transform light energy into chemical energy by a process unrelated to chlorophyll-based photosynthesis.
Haloarchaea have a potential to solubilize phosphorus. Phosphorus-solubilizing halophilic archaea may well play a role in P (phosphorus) nutrition to vegetation growing in hypersaline soils. Haloarchaea may also have applications as inoculants for crops growing in hypersaline regions.[6]
Taxonomy
The extremely halophilic, aerobic members of Archaea are classified within the family Halobacteriaceae, order Halobacteriales in Class III. Halobacteria of the phylum Euryarchaeota (International Committee on Systematics of Prokaryotes, Subcommittee on the taxonomy of Halobacteriaceae). As of May 2016, the family Halobacteriaceae comprises 213 species in 50 genera.
| Species list |
| Domain: Archaea
Euryarchaeota
non-valid |
Classification of Gupta et al.[15][16]
Halobacteriales
- Halobacteriaceae (Type genera: Halobacterium)
Haladaptatus, Halalkalicoccus, Haloarchaeobius, Halarchaeum, Halobacterium, Halocalculus, Halorubellus, Halorussus, "Halosiccatus", Halovenus, Natronoarchaeum, Natronomonas, Salarchaeum.
- Haloarculaceae (Type genera: Haloarcula)
Halapricum, Haloarcula, Halomicroarcula, Halomicrobium, Halorientalis, Halorhabdus, Halosimplex.
- Halococcaceae (Type genera: Halococcus)
Halococcus.
Haloferacales
- Haloferacaceae (Type genera: Haloferax)
Halabellus, Haloferax, Halogeometricum, (Halogranum), Halopelagius, Haloplanus, Haloquadratum, Halosarcina.
- Halorubraceae (Type genera: Halorubrum)
Halobaculum, (Halogranum), Halohasta, Halolamina, Halonotius, Halopenitus, Halorubrum, Salinigranum.
Natrialbales
- Natrialbaceae (Type genera: Natrialba)
Halobiforma, Halopiger, Halostagnicola, Haloterrigena, Halovarius, Halovivax, Natrialba, Natribaculum, Natronobacterium, Natronococcus, Natronolimnobius, Natronorubrum, Salinarchaeum.
Living environment

Haloarchaea require salt concentrations in excess of 2 M (or about 10%) to grow, and optimal growth usually occurs at much higher concentrations, typically 20–25%. However, Haloarchaea can grow up to saturation (about 37% salts).[17]
Haloarchaea are found mainly in hypersaline lakes and solar salterns. Their high densities in the water often lead to pink or red colourations of the water (the cells possessing high levels of carotenoid pigments, presumably for UV protection).[18] Some of them live in underground rock salt deposits, including one from middle-late Eocene (38-41 million years ago).[19] Some even older ones from more than 250 million years ago have been reported.[20]
Adaptations to environment
Haloarchaea can grow at an aw close to 0.75, yet a water activity (aw) lower than 0.90 is inhibitory to most microbes.[21] The number of solutes causes osmotic stress on microbes, which can cause cell lysis, unfolding of proteins and inactivation of enzymes when there is a large enough imbalance.[22] Haloarchaea combat this by retaining compatible solutes such as potassium chloride (KCl) in their intracellular space to allow them to balance osmotic pressure.[23] Retaining these salts is referred to as the “salt-in” method where the cell accumulates a high internal concentration of potassium.[24] Because of the elevated potassium levels, haloarchaea have specialized proteins that have a highly negative surface charge to tolerate high potassium concentrations.[25]
Haloarchaea have adapted to use glycerol as a carbon and energy source in catabolic processes, which is often present in high salt environments due to Dunaliella species that produce glycerol in large quantities.[24]
Phototrophy
Bacteriorhodopsin is used to absorb light, which provides energy to transport protons (H+) across the cellular membrane. The concentration gradient generated from this process can then be used to synthesize ATP. Many haloarchaea also possess related pigments, including halorhodopsin, which pumps chloride ions in the cell in response to photons, creating a voltage gradient and assisting in the production of energy from light. The process is unrelated to other forms of photosynthesis involving electron transport, however, and haloarchaea are incapable of fixing carbon from carbon dioxide.[26] Early evolution of retinal proteins has been proposed as the purple Earth hypothesis.[5]
Cellular shapes
Haloarchaea are often considered pleomorphic, or able to take on a range of shapes—even within a single species. This makes identification by microscopic means difficult, and it is now more common to use gene sequencing techniques for identification instead.
One of the more unusually shaped Haloarchaea is the "Square Haloarchaeon of Walsby". It was classified in 2004 using a very low nutrition solution to allow growth along with a high salt concentration, square in shape and extremely thin (like a postage stamp). This shape is probably only permitted by the high osmolarity of the water, permitting cell shapes that would be difficult, if not impossible, under other conditions.
As exophiles
Haloarchaea have been proposed as a kind of life that could live on Mars; since the Martian atmosphere has a pressure below the triple point of water, freshwater species would have no habitat on the Martian surface. The presence of high salt concentrations in water lowers its freezing point, in theory allowing for halophiles to exist in saltwater on Mars.[27]
See also
- Life on Mars
- Purple Earth hypothesis
References
- Fendrihan S, Legat A, Pfaffenhuemer M, Gruber C, Weidler G, Gerbl F, Stan-Lotter H (August 2006). "Extremely halophilic archaea and the issue of long-term microbial survival". Re/Views in Environmental Science and Bio/Technology. 5 (2–3): 203–218. doi:10.1007/s11157-006-0007-y. PMC 3188376. PMID 21984879.
- See the NCBI webpage on Halobacteria. Data extracted from the "NCBI taxonomy resources". National Center for Biotechnology Information. Retrieved 2007-03-19.
- DasSarma P, DasSarma S (May 2008). "On the origin of prokaryotic "species": the taxonomy of halophilic Archaea". Saline Systems. 4 (1): 5. doi:10.1186/1746-1448-4-5. PMC 2397426. PMID 18485204.
- DasSarma S, DasSarma P (2017). Halophiles. eLS. John Wiley & Sons, Ltd. pp. 1–13. doi:10.1002/9780470015902.a0000394.pub4. ISBN 9780470015902.
- DasSarma S, Schwieterman EW (2018). "Early evolution of purple retinal pigments on Earth and implications for exoplanet biosignatures". International Journal of Astrobiology: 1–10. arXiv:1810.05150. doi:10.1017/S1473550418000423. ISSN 1473-5504.
- Yadav AN, Sharma D, Gulati S, Singh S, Dey R, Pal KK, et al. (July 2015). "Haloarchaea Endowed with Phosphorus Solubilization Attribute Implicated in Phosphorus Cycle". Scientific Reports. 5: 12293. Bibcode:2015NatSR...512293Y. doi:10.1038/srep12293. PMC 4516986. PMID 26216440.
- Minegishi H, Echigo A, Shimane Y, Kamekura M, Tanasupawat S, Visessanguan W, Usami R (September 2012). "Halobacterium piscisalsi Yachai et al. 2008 is a later heterotypic synonym of Halobacterium salinarum Elazari-Volcani 1957". International Journal of Systematic and Evolutionary Microbiology. 62 (Pt 9): 2160–2. doi:10.1099/ijs.0.036905-0. PMID 22058320.
- Ugalde JA, Narasingarao P, Kuo S, Podell S, Allen EE (December 2013). "Draft Genome Sequence of "Candidatus Halobonum tyrrellensis" Strain G22, Isolated from the Hypersaline Waters of Lake Tyrrell, Australia". Genome Announcements. 1 (6). doi:10.1128/genomeA.01001-13. PMC 3861417. PMID 24336364.
- Filker S, Kaiser M, Rosselló-Móra R, Dunthorn M, Lax G, Stoeck T (June 2014). ""Candidatus Haloectosymbiotes riaformosensis" (Halobacteriaceae), an archaeal ectosymbiont of the hypersaline ciliate Platynematum salinarum". Systematic and Applied Microbiology. 37 (4): 244–51. doi:10.1016/j.syapm.2014.01.001. hdl:10261/99984. PMID 24629416.
- Hassani II, Robert C, Michelle C, Raoult D, Hacène H, Desnues C (October 2013). "Non-contiguous finished genome sequence and description of Halopiger djelfamassiliensis sp. nov". Standards in Genomic Sciences. 9 (1): 160–74. doi:10.4056/sigs.4578289. PMC 3910545. PMID 24501653.
- Ikram HI, Catherine R, Caroline M, Didier R, Hocine H, Christelle D (June 2014). "Non-contiguous finished genome sequence and description of Halopiger goleamassiliensis sp. nov". Standards in Genomic Sciences. 9 (3): 956–9. doi:10.4056/sigs.4618288. PMC 4149028. PMID 25197441.
- Sánchez-Nieves R, Facciotti MT, Saavedra-Collado S, Dávila-Santiago L, Rodríguez-Carrero R, Montalvo-Rodríguez R (March 2016). "Draft genome sequence of Halorubrum tropicale strain V5, a novel halophilic archaeon isolated from the solar salterns of Cabo Rojo, Puerto Rico". Genomics Data. 7: 284–6. doi:10.1016/j.gdata.2016.02.004. PMC 4778665. PMID 26981427.
- Sánchez-Nieves R, Facciotti M, Saavedra-Collado S, Dávila-Santiago L, Rodríguez-Carrero R, Montalvo-Rodríguez R (March 2016). "Draft genome of Haloarcula rubripromontorii strain SL3, a novel halophilic archaeon isolated from the solar salterns of Cabo Rojo, Puerto Rico". Genomics Data. 7: 287–9. doi:10.1016/j.gdata.2016.02.005. PMC 4778667. PMID 26981428.
- Jaakkola ST, Pfeiffer F, Ravantti JJ, Guo Q, Liu Y, Chen X, Ma H, Yang C, Oksanen HM, Bamford DH (February 2016). "The complete genome of a viable archaeum isolated from 123-million-year-old rock salt". Environmental Microbiology. 18 (2): 565–79. doi:10.1111/1462-2920.13130. PMID 26628271.
- Gupta RS, Naushad S, Baker S (March 2015). "Phylogenomic analyses and molecular signatures for the class Halobacteria and its two major clades: a proposal for division of the class Halobacteria into an emended order Halobacteriales and two new orders, Haloferacales ord. nov. and Natrialbales ord. nov., containing the novel families Haloferacaceae fam. nov. and Natrialbaceae fam. nov". International Journal of Systematic and Evolutionary Microbiology. 65 (Pt 3): 1050–69. doi:10.1099/ijs.0.070136-0. PMID 25428416.
- Gupta RS, Naushad S, Fabros R, Adeolu M (April 2016). "A phylogenomic reappraisal of family-level divisions within the class Halobacteria: proposal to divide the order Halobacteriales into the families Halobacteriaceae, Haloarculaceae fam. nov., and Halococcaceae fam. nov., and the order Haloferacales into the families, Haloferacaceae and Halorubraceae fam nov". Antonie van Leeuwenhoek. 109 (4): 565–87. doi:10.1007/s10482-016-0660-2. PMID 26837779.
- Yadav AN, Sharma D, Gulati S, Singh S, Dey R, Pal KK, et al. (July 2015). "Haloarchaea Endowed with Phosphorus Solubilization Attribute Implicated in Phosphorus Cycle". Scientific Reports. 5: 12293. Bibcode:2015NatSR...512293Y. doi:10.1038/srep12293. PMC 4516986. PMID 26216440.
- DasSarma, Shiladitya (2007). "Extreme Microbes". American Scientist. 95 (3): 224–231. doi:10.1511/2007.65.1024. ISSN 0003-0996.
- Jaakkola ST, Zerulla K, Guo Q, Liu Y, Ma H, Yang C, et al. (2014). "Halophilic archaea cultivated from surface sterilized middle-late eocene rock salt are polyploid". PLOS ONE. 9 (10): e110533. Bibcode:2014PLoSO...9k0533J. doi:10.1371/journal.pone.0110533. PMC 4206341. PMID 25338080.
- Vreeland RH, Rosenzweig WD, Lowenstein T, Satterfield C, Ventosa A (February 2006). "Fatty acid and DNA analyses of Permian bacteria isolated from ancient salt crystals reveal differences with their modern relatives". Extremophiles. 10 (1): 71–8. doi:10.1007/s00792-005-0474-z. PMID 16133658.
- Stevenson A, Cray JA, Williams JP, Santos R, Sahay R, Neuenkirchen N, et al. (June 2015). "Is there a common water-activity limit for the three domains of life?". The ISME Journal. 9 (6): 1333–51. doi:10.1038/ismej.2014.219. PMC 4438321. PMID 25500507.
- Cheftel JC (1 August 1995). "Review : High-pressure, microbial inactivation and food preservation". Food Science and Technology International. 1 (2–3): 75–90. doi:10.1177/108201329500100203.
- da Costa MS, Santos H, Galinski EA (1998). Biotechnology of Extremophiles. Advances in Biochemical Engineering/Biotechnology. 61. Springer, Berlin, Heidelberg. pp. 117–153. doi:10.1007/bfb0102291. ISBN 978-3-540-63817-9.
- Williams TJ, Allen M, Tschitschko B, Cavicchioli R (March 2017). "Glycerol metabolism of haloarchaea". Environmental Microbiology. 19 (3): 864–877. doi:10.1111/1462-2920.13580. PMID 27768817.
- Soppa J, Baumann A, Brenneis M, Dambeck M, Hering O, Lange C (September 2008). "Genomics and functional genomics with haloarchaea". Archives of Microbiology. 190 (3): 197–215. doi:10.1007/s00203-008-0376-4. PMID 18493745.
- Bryant DA, Frigaard NU (November 2006). "Prokaryotic photosynthesis and phototrophy illuminated". Trends in Microbiology. 14 (11): 488–96. doi:10.1016/j.tim.2006.09.001. PMID 16997562.
- DasSarma S (2006). "Extreme halophiles are models for astrobiology" (PDF). Microbe-American Society for Microbiology. 1 (3): 120. Archived from the original (PDF) on 2007-02-02.
Further reading
Journals
- Soppa J (March 2006). "From genomes to function: haloarchaea as model organisms". Microbiology. 152 (Pt 3): 585–90. doi:10.1099/mic.0.28504-0. PMID 16514139.
- Cavalier-Smith T (January 2002). "The neomuran origin of archaebacteria, the negibacterial root of the universal tree and bacterial megaclassification". International Journal of Systematic and Evolutionary Microbiology. 52 (Pt 1): 7–76. doi:10.1099/00207713-52-1-7. PMID 11837318.
- Woese CR, Kandler O, Wheelis ML (June 1990). "Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya". Proceedings of the National Academy of Sciences of the United States of America. 87 (12): 4576–9. Bibcode:1990PNAS...87.4576W. doi:10.1073/pnas.87.12.4576. PMC 54159. PMID 2112744.
- Cavalier-Smith T (1986). "The kingdoms of organisms". Nature. 324 (6096): 416–7. Bibcode:1986Natur.324..416C. doi:10.1038/324416a0. PMID 2431320.
Books
- Grant WD, Kamekura M, McGenity TJ, Ventosa A (2001). "Class III. Halobacteria class. nov.". In DR Boone, RW Castenholz (eds.). Bergey's Manual of Systematic Bacteriology Volume 1: The Archaea and the deeply branching and phototrophic Bacteria (2nd ed.). New York: Springer Verlag. pp. 169. ISBN 978-0-387-98771-2.
- Garrity GM, Holt JG (2001). "Phylum AII. Euryarchaeota phy. nov.". In DR Boone, RW Castenholz (eds.). Bergey's Manual of Systematic Bacteriology Volume 1: The Archaea and the deeply branching and phototrophic Bacteria (2nd ed.). New York: Springer Verlag. pp. 169. ISBN 978-0-387-98771-2.