Mosquito-borne disease
Mosquito-borne diseases or mosquito-borne illnesses are diseases caused by bacteria, viruses or parasites transmitted by mosquitoes. Nearly 700 million people get a mosquito-borne illness each year resulting in over one million deaths.[1]
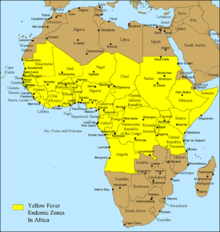
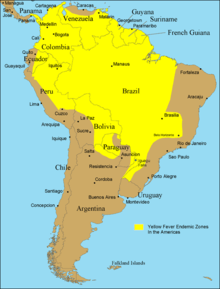
Diseases transmitted by mosquitoes include malaria, dengue, West Nile virus, chikungunya, yellow fever,[1] filariasis, tularemia, dirofilariasis, Japanese encephalitis, Saint Louis encephalitis, Western equine encephalitis, Eastern equine encephalitis,[2] Venezuelan equine encephalitis, Ross River fever, Barmah Forest fever, La Crosse encephalitis, and Zika fever,[2] as well as newly detected Keystone virus and Rift Valley fever. There is no evidence as of April 2020 that COVID-19 can be transmitted by mosquitoes, and it is extremely unlikely this could occur.[3][4] Also HIV/AIDS is not transmittable through mosquito contact, despite being caused by a virus that can be transmitted through blood.[5]
Types
Protozoa
The female mosquito of the genus Anopheles may carry the malaria parasite. Four different species of protozoa cause malaria: Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale and Plasmodium vivax[6] (see Plasmodium). Worldwide, malaria is a leading cause of premature mortality, particularly in children under the age of five, with an estimated 207 million cases and more than half a million deaths in 2012, according to the World Malaria Report 2013 published by WHO. The death toll increased to one million as of 2018 according to the American Mosquito Control Association.[7]
Myiasis
Botflies are known to parasitize humans or other mammalians, causing myiasis, and to use mosquitoes as intermediate vector agents to deposit eggs on a host. The human botfly Dermatobia hominis attaches its eggs to the underside of a mosquito, and when the mosquito takes a blood meal from a human or an animal, the body heat of the mammalian host induces hatching of the larvae.
Helminthiasis
Some species of mosquito can carry the filariasis worm, a parasite that causes a disfiguring condition (often referred to as elephantiasis) characterized by a great swelling of several parts of the body; worldwide, around 40 million people are living with a filariasis disability.
Virus
The viral diseases yellow fever, dengue fever, Zika fever and chikungunya are transmitted mostly by Aedes aegypti mosquitoes.
Other viral diseases like epidemic polyarthritis, Rift Valley fever, Ross River fever, St. Louis encephalitis, West Nile fever, Japanese encephalitis, La Crosse encephalitis and several other encephalitic diseases are carried by several different mosquitoes. Eastern equine encephalitis (EEE) and Western equine encephalitis (WEE) occur in the United States where they cause disease in humans, horses, and some bird species. Because of the high mortality rate, EEE and WEE are regarded as two of the most serious mosquito-borne diseases in the United States. Symptoms range from mild flu-like illness to encephalitis, coma and death.[8]
Viruses carried by arthropods such as mosquitoes or ticks are known collectively as arboviruses. West Nile virus was accidentally introduced into the United States in 1999 and by 2003 had spread to almost every state with over 3,000 cases in 2006.
Other species of Aedes as well as Culex and Culiseta are also involved in the transmission of disease.
Myxomatosis is spread by biting insects, including mosquitoes.[9]
Transmission
A mosquito's period of feeding is often undetected; the bite only becomes apparent because of the immune reaction it provokes. When a mosquito bites a human, it injects saliva and anti-coagulants. For any given individual, with the initial bite there is no reaction but with subsequent bites the body's immune system develops antibodies and a bite becomes inflamed and itchy within 24 hours. This is the usual reaction in young children. With more bites, the sensitivity of the human immune system increases, and an itchy red hive appears in minutes where the immune response has broken capillary blood vessels and fluid has collected under the skin. This type of reaction is common in older children and adults. Some adults can become desensitized to mosquitoes and have little or no reaction to their bites, while others can become hyper-sensitive with bites causing blistering, bruising, and large inflammatory reactions, a response known as skeeter syndrome.
Signs and symptoms
Symptoms of illness are specific to the type of viral infection and vary on severity, based on the individuals infected.
Zika virus
Symptoms vary on severity, from mild unnoticeable symptoms to more common symptoms like fever, rash, headache, achy muscle and joints, and conjunctivitis. Symptoms can last several days to weeks, but death resulting from this infection is rare.[10]
West Nile virus, dengue fever
Most people infected with the West Nile virus usually do not develop symptoms. However, some individuals can develop cases of severe fatigue, weakness, headaches, body aches, joint and muscle pain, vomiting, diarrhea, and rash, which can last for weeks or months. More serious symptoms have a greater risk of appearing in people over 60 years of age, or those suffering from cancer, diabetes, hypertension, and kidney disease.[11]
Dengue fever is mostly characterized by high fever, headaches, joint pain, and rash. However, more severe instances can lead to hemorrhagic fever, internal bleeding, and breathing difficulty, which can be fatal.[12]
Chikungunya
People infected with this virus can develop sudden onset fever along with debilitating joint and muscle pain, rash, headache, nausea, and fatigue. Symptoms can last a few days or be prolonged to weeks and months. Although patients can recover completely, there have been cases in which joint pain has persisted for several months and can extend beyond that for years. Other people can develop heart complications, eye problems, and even neurological complications.[13]
Mechanism
Mosquitoes carrying such arboviruses stay healthy because their immune systems recognizes the virions as foreign particles and "chop off" the virus' genetic coding, rendering it inert. Human infection with a mosquito-borne virus occurs when a female mosquito bites someone while its immune system is still in the process of destroying the virus's harmful coding.[14] It is not completely known how mosquitoes handle eukaryotic parasites to carry them without being harmed. Data has shown that the malaria parasite Plasmodium falciparum alters the mosquito vector's feeding behavior by increasing frequency of biting in infected mosquitoes, thus increasing the chance of transmitting the parasite.[15]
The mechanism of transmission of this disease starts with the injection of the parasite into the victim's blood when malaria-infected female Anopheles mosquitoes bite into a human being. The parasite uses human liver cells as hosts for maturation where it will continue to replicate and grow, moving into other areas of the body via the bloodstream. The spread of this infection cycle then continues when other mosquitoes bite the same victim. The result will cause that mosquito to ingest the parasite and allow it to transmit the Malaria disease into another person through the same mode of bite injection.[16]
Other Flaviviridae viruses transmissible via vectors like mosquitoes include West Nile virus and yellow fever virus, which are single stranded, positive-sense RNA viruses enveloped in a protein coat. Once inside the host's body, the virus will attach itself to a cell's surface through receptor-mediated endocytosis. This essentially means that the proteins and DNA material of the virus are ingested into the host cell. The viral RNA material will undergo several changes and processes inside the host's cell so that it can release more viral RNA that can then be replicated and assembled to infect neighboring host cells.[17] The data on transmissibility via insect vectors of hepatitis C virus, also belonging to family Flaviviridae (as well as for hepatitis B virus, belonging to family Hepadnaviridae) are inconclusive. WHO states that "There is no insect vector or animal reservoir for HCV.",[18] while there are experimental data supporting at least the presence of [PCR]-detectable hepatitis C viral RNA in Culex mosquitoes for up to 13 days.[19]
Currently, there are no specific vaccine therapies for West Nile virus approved for humans; however, vaccines are available and some show promising for animals, as a means to intervene with the mechanism of spreading such pathogens.[20]
Diagnosis
Doctors can typically identify a mosquito bite by sight.[21]
A doctor will perform a physical examination and ask about medical history as well as any travel history.[21] Be ready to give details on any international trips, including the dates you were traveling, the countries you visited and any contact you had with mosquitoes.
Dengue fever
Diagnosing dengue fever can be difficult, as its symptoms often overlap with many other diseases such as malaria and typhoid fever.[22] Laboratory tests can detect evidence of the dengue viruses, however the results often come back too late to assist in directing treatment.[22]
West Nile virus
Medical testing can confirm the presence of West Nile fever or a West Nile-related illness, such as meningitis or encephalitis.[23] If infected, a blood test may show a rising level of antibodies to the West Nile virus. A lumbar puncture (spinal tap) is the most common way to diagnose meningitis, by analyzing the cerebrospinal fluid surrounding your brain and spinal cord.[24] The fluid sample may show an elevated white cell count and antibodies to the West Nile virus if you were exposed.[24] In some cases, an electroencephalography (EEG) or magnetic resonance imaging (MRI) scan can help detect brain inflammation.[24]
Zika virus
A Zika virus infection might be suspected if symptoms are present and an individual has traveled to an area with known Zika virus transmission.[25] Zika virus can only be confirmed by a laboratory test of body fluids, such as urine or saliva, or by blood test.[25]
Chikungunya
Laboratory blood tests can identify evidence of chikungunya or other similar viruses such as dengue and Zika.[26] Blood test may confirm the presence of IgM and IgG anti-chikungunya antibodies. IgM antibodies are highest 3 to 5 weeks after the beginning of symptoms and will continue be present for about 2 months.[26]
Prevention
There is a re-emergence of mosquito vector viruses (arthropod-borne viruses) called arboviruses carried by the Aedes aegypti mosquito. Examples are the Zika virus, chikungunya virus, yellow fever and dengue fever. The re-emergence of the viruses has been at a faster rate, and over a wider geographic area, than in the past. The rapid re-emergence is due to expanding global transportation networks, the mosquito's increasing ability to adapt to urban settings, the disruption of traditional land use and the inability to control expanding mosquito populations.[27] Like malaria, other arboviruses do not have a vaccine. The only exception is yellow fever. Prevention is focused on reducing the adult mosquito populations, controlling mosquito larvae and protecting individuals from mosquito bites. Depending on the mosquito vector, and the affected community, a variety of prevention methods may be deployed at one time.
Insecticidal nets and indoor residual spraying
The use of insecticide treated mosquito nets (ITNs) are at the forefront of preventing mosquito bites that cause malaria. The prevalence of ITNs in sub-Saharan Africa has grown from 3% of households to 50% of households from 2000 to 2010 with over 254 million insecticide treated nets distributed throughout sub-Saharan Africa for use against the mosquito vectors Anopheles gambiae and Anopheles funestus which carry malaria. Because the Anopheles gambiae feeds indoors (endophagic) and rests indoors after feeding (endophilic), insecticide treated nets (ITNs) interrupt the mosquito's feeding pattern. The ITNs continue to offer protection, even after there are holes in the nets, because of their excito-repellency properties which reduce the number of mosquitoes that enter the home. The World Health Organization (WHO) recommends treating ITNs with the pyrethroid class of insecticides. There is an emerging concern of mosquito resistance to insecticides used in ITNs. Twenty-seven (27) sub-Saharan African countries have reported Anopheles vector resistance to pyrethroid insecticides.[28]
Indoor spraying of insecticides is another prevention method widely used to control mosquito vectors. To help control the Aedes aegypti mosquito, homes are sprayed indoors with residual insecticide applications. Indoor residual spraying (IRS) reduces the female mosquito population and mitigates the risk of dengue virus transmission. Indoor residual spraying is completed usually once or twice a year. Mosquitoes rest on walls and ceilings after feeding and are killed by the insecticide. Indoor spraying can be combined with spraying the exterior of the building to help reduce the number of mosquito larvae and subsequently, the number of adult mosquitoes.[29]
Personal protection methods
There are other methods that an individual can use to protect themselves from mosquito bites. Limiting exposure to mosquitoes from dusk to dawn when the majority of mosquitoes are active and wearing long sleeves and long pants during the period mosquitoes are most active. Placing screens on windows and doors is a simple and effective means of reducing the number of mosquitoes indoors. Anticipating mosquito contact and using a topical mosquito repellant with DEET or icaridin is also recommended. Draining or covering water receptacles, both indoor and outdoors, is also a simple but effective prevention method. Removing debris and tires, cleaning drains, and cleaning gutters help larval control and reduce the number of adult mosquitoes.[30]
Vaccines
There is a vaccine for yellow fever which was developed in the 1930s, the yellow 17D vaccine, and it is still in use today. The initial yellow fever vaccination provides lifelong protection for most people and provides immunity within 30 days of the vaccine. Reactions to the yellow fever vaccine have included mild headache and fever, and muscle aches. There are rare cases of individuals presenting with symptoms that mirror the disease itself. The risk of complications from the vaccine are greater for individuals over 60 years of age. In addition, the vaccine is not usually administered to babies under nine months of age, pregnant women, people with allergies to egg protein, and individuals living with AIDS/HIV. The World Health Organization (WHO) reports that 105 million people have been vaccinated for yellow fever in West Africa from 2000 to 2015.[31]
To date, there are relatively few vaccines against mosquito-borne diseases. The National Institute of Allergy and Infectious Disease (NIAID) began Phase 1 clinical trials of a new vaccine that would be nearly universal in protecting against the majority of mosquito-borne diseases.[32]
Education and community involvement
The arboviruses have expanded their geographic range and infected populations that had no recent community knowledge of the diseases carried by the Aedes aegypti mosquito. Education and community awareness campaigns are necessary for prevention to be effective. Communities are educated on how the disease is spread, how they can protect themselves from infection and the symptoms of infection.[30] Community health education programs can identify and address the social/economic and cultural issues that can hinder preventative measures. Community outreach and education programs can identify which preventative measures a community is most likely to employ. Leading to a targeted prevention method that has a higher chance of success in that particular community. Community outreach and education includes engaging community health workers and local healthcare providers, local schools and community organizations to educate the public on mosquito vector control and disease prevention.[33]
Treatments
Yellow fever
Numerous drugs have been used to treat yellow fever disease with minimal satisfaction to date. Patients with multisystem organ involvement will require critical care support such as possible hemodialysis or mechanical ventilation. Rest, fluids, and acetaminophen are also known to relieve milder symptoms of fever and muscle pain. Due to hemorrhagic complications, aspirin should be avoided. Infected individuals should avoid mosquito exposure by staying indoors or using a mosquito net.[34]
Dengue fever
Dengue infection's therapeutic management is simple, cost effective and successful in saving lives by adequately performing timely institutionalized interventions. Treatment options are restricted, while no effective antiviral drugs for this infection have been accessible to date. Patients in the early phase of the dengue virus may recover without hospitalization. However, ongoing clinical research is in the works to find specific anti-dengue drugs.[35]
Zika virus
Zika virus vaccine clinical trials are to be conducted and established. There are efforts being put toward advancing antiviral therapeutics against zika virus for swift control. Present day Zika virus treatment is symptomatic through antipyretics and analgesics. Currently there are no publications regarding viral drug screening. Nevertheless, therapeutics for this infection have been used.[36]
Chikungunya
There are no treatment modalities for acute and chronic chikungunya that currently exist. Most treatment plans use supportive and symptomatic care like analgesics for pain and anti-inflammatories for inflammation caused by arthritis. In acute stages of this virus, rest, antipyretics and analgesics are used to subside symptoms. Most use non-steroidal anti-inflammatory drugs (NSAIDs). In some cases, joint pain may resolve from treatment but stiffness remains.[37]
Latest treatment
The sterile insect technique (SIT) uses irradiation to sterilize insect pests before releasing them in large numbers to mate with wild females. Since they do not produce any offspring, the population, and consequently the disease incidence, is reduced over time. Used successfully for decades to combat fruit flies and livestock pests such as screwworm and tsetse flies, the technique can be adapted also for some disease-transmitting mosquito species. Pilot projects are being initiated or are under way in different parts of the world.[38][39]
Epidemiology
Mosquito-borne diseases, such as dengue fever and malaria, typically affect developing countries and areas with tropical climates. Mosquito vectors are sensitive to climate changes and tend to follow seasonal patterns. Between years there are often dramatic shifts in incidence rates. The occurrence of this phenomenon in endemic areas makes mosquito-borne viruses difficult to treat.[40]
Dengue fever is caused by infection through viruses of the family Flaviviridae. The illness is most commonly transmitted by Aedes aegypti mosquitoes in tropical and subtropical regions.[41] Dengue virus has four different serotypes, each of which are antigenically related but have limited cross-immunity to reinfection.[42]
Although dengue fever has a global incidence of 50-100 million cases, only several hundreds of thousands of these cases are life-threatening. The geographic prevalence of the disease can be examined by the spread of the Aedes aegypti.[43] Over the last twenty years, there has been a geographic spread of the disease. Dengue incidence rates have risen sharply within urban areas which have recently become endemic hot spots for the disease.[44] The recent spread of Dengue can also be attributed to rapid population growth, increased coagulation in urban areas, and global travel. Without sufficient vector control, the dengue virus has evolved rapidly over time, posing challenges to both government and public health officials.
Malaria is caused by a protozoan called Plasmodium falciparum. P. falciparum parasites are transmitted mainly by the Anopheles gambiae complex in rural Africa.[41] In just this area, P. falciparum infections comprise an estimated 200 million clinical cases and 1 million annual deaths. 75% of individuals afflicted in this region are children.[44] As with dengue, changing environmental conditions have led to novel disease characteristics. Due to increased illness severity, treatment complications, and mortality rates, many public health officials concede that malaria patterns are rapidly transforming in Africa.[45] Scarcity of health services, rising instances of drug resistance, and changing vector migration patterns are factors that public health officials believe contribute to malaria’s dissemination.
Climate heavily affects mosquito vectors of malaria and dengue. Climate patterns influence the lifespan of mosquitos as well as the rate and frequency of reproduction. Climate change impacts have been of great interest to those studying these diseases and their vectors. Additionally, climate impacts mosquito blood feeding patterns as well as extrinsic incubation periods.[41] Climate consistency gives researchers an ability to accurately predict annual cycling of the disease but recent climate unpredictability has eroded researchers’ ability to track the disease with such precision.
Advances in biological control of arboviruses
In many insect species, such as Drosophila melanogaster, researchers found that a natural infection with the bacteria strain Wolbachia pipientis increases the fitness of the host by increasing resistance to RNA viral infections.[46] Robert L. Glaser and Mark A. Meola investigated Wolbachia-induced resistance to West Nile virus (WNV) in Drosophila melanogaster (fruit flies).[46] Two groups of fruit flies were naturally infected with Wolbachia. Glaser and Meola then cured one group of fruit flies of Wolbachia using tetracycline. Both the infected group and the cured groups were then infected with WNV. Flies infected with Wolbachia were found to have a changed phenotype that caused resistance to WNV. The phenotype was found to be caused by a “dominant, maternally transmitted, cytoplasmic factor”.[46] The WNV-resistance phenotype was then reversed by curing the fruit flies of Wolbachia. Since Wolbachia is also maternally transmitted, it was found that the WNV-resistant phenotype is directly related to the Wolbachia infection.[46] West Nile virus is transmitted to humans and animals through the Southern house mosquito, Culex quinquefasciatus. Glaser and Meola knew vector compatibility could be reduced through Wolbachia infection due to studies done with other species of mosquitoes, mainly, Aedes aegypti. Their goal was to transfer WNV resistance to Cx. quinquefasciatus by inoculating the embryos of the mosquito with the same strain of Wolbachia that naturally occurred in the fruit flies. Upon infection, Cx. quinquefasciatus showed an increased resistance to WNV that was transferable to offspring.[46] The ability to genetically modify mosquitoes in the lab and then have the infected mosquitoes transmit it to their offspring showed that it was possible to transmit the bacteria to wild populations to decrease human infections.
In 2011, Ary Hoffmann and associates produced the first case of Wolbachia-induced arbovirus resistance in wild populations of Aedes aegypti through a small project called Eliminate Dengue: Our Challenge.[47] This was made possible by an engineered strain of Wolbachia termed wMel that came from D. melanogaster. The transfer of wMel from D. melanogaster into field-caged populations of the mosquito Aedes aegypti induced resistance to dengue, yellow fever, and chikungunya viruses. Although other strains of Wolbachia also reduced susceptibility to dengue infection, they also put a greater demand on the fitness of Ae. aegypti. wMel was different in that it was thought to only cost the organism a small portion of its fitness.[47][48] wMel-infected Ae. aegypti were released into two residential areas in the city of Cairns, Australia over a 14-week period. Hoffmann and associates, released a total of 141,600 infected adult mosquitoes in Yorkeys Knob suburb and 157,300 in Gordonvale suburb.[47] After release, the populations were monitored for three years to record the spread of wMel. Population monitoring was gauged by measuring larvae laid in traps. At the beginning of the monitoring period but still within the release period, it was found that wMel-infected Ae. aegypti had doubled in Yorkeys Knob and increased 1.5-fold in Gordonvale.[47][48] Uninfected Ae. aegypti populations were in decline. By the end of the three years, wMel-infected Ae. aegypti had stable populations of about 90%. However, these populations were isolated to the Yorkeys Knob and Gordonvale suburbs due to unsuitable habitat surrounding the neighborhoods.[48]
Although populations flourished in these areas with nearly 100% transmission, no signs of spread were noted, proving disappointing for some.[49] Following this experiment, Tom L. Schmidt and his colleagues conducted an experiment releasing Wolbachia-infected Aedes aegypti using different site selection methods occurred in different areas of Cairns during 2013. The release sites were monitored over two years. This time the release was done in urban areas that were adjacent to adequate habitat to encourage mosquito dispersal. Over the two years, the population doubled, and spatial spread was also increased, unlike the first release,[49] giving ample satisfactory results. By increasing the spread of the Wolbachia-infected mosquitoes, the researchers were able to establish that population of a large city was possible if the mosquitoes were given adequate habitat to spread into upon release in different local locations throughout the city.[49] An important detail in both of these studies is that no adverse effects on public health or the natural ecosystem occurred.[50] This made it an extremely attractive alternative to traditional insecticide methods given the increased pesticide resistance occurring from heavy use.
From the success seen in Australia, the researchers were able to begin operating in more threatened portions of the world. The Eliminate Dengue program spread to 10 countries throughout Asia, Latin America, and the Western Pacific blooming into the non-profit organization, World Mosquito Program, as of September 2017.[50] They still use the same technique of infecting wild populations of Ae. aegypti as they did in Australia, but this time their target disease has shifted to include Zika and chikungunya on top of dengue.[50] Although not alone in their efforts to use Wolbachia-infected mosquitoes to reduce mosquito-borne disease, the World Mosquito Program method is praised for being self-sustaining in that it causes permanent phenotype change rather than reducing mosquito populations through cytoplasmic incompatibility through male-only dispersal.[50]
References
- Caraballo, Hector (May 2014). "Emergency Department Management Of Mosquito-Borne Illness: Malaria, Dengue, And West Nile Virus". Emergency Medicine Practice. 16 (5): 1–23, quiz 23–4. PMID 25207355.
- "Diseases that can be Transmitted by Mosquitoes - Minnesota Dept. of Health". www.health.state.mn.us. Retrieved 2018-02-15.
- "WHO | Myth busters". www.who.int. Retrieved 2020-04-18.
- Service, Purdue News. "It's extremely unlikely mosquitoes can transmit COVID-19, Purdue professor says". www.purdue.edu. Retrieved 2020-05-12.
- "Can I get HIV from mosquitoes?". CDC. October 20, 2006. Archived from the original on April 2, 2016.
- "WHO | Malaria". www.who.int. Retrieved 2018-02-15.
- "Mosquito-Borne Diseases - American Mosquito Control Association". www.mosquito.org. Retrieved 2018-02-15.
- "Mosquito-borne diseases, infectious disease information, NCID, CDC". Retrieved 20 August 2019.
- Kerr, Peter (2013). "Viral Infections of Rabbits". Veterinary Clinics of North America: Exotic Animal Practice. 16 (2): 437–468. doi:10.1016/j.cvex.2013.02.002. PMC 7110462. PMID 23642871.
- "Zika Symptoms". CDC. 2017-08-30. Retrieved 2017-09-16.
- "Symptoms, Diagnosis, & Treatment | West Nile Virus | CDC". www.cdc.gov. 2017-08-02. Retrieved 2017-09-16.
- "Mosquito-Borne Diseases". Baylor College of Medicine. Retrieved 2017-09-16.
- "Chikungunya". World Health Organization. Retrieved 2017-09-16.
- Susannah F Locke (1 December 2008). "Bug vs Bug: How do mosquitoes survive deadly viruses unscathed?".
- Koella, J.C.; Sorensen; Anderson (7 May 1998). "The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae". Proceedings of the Royal Society B. 265 (1398): 763–768. doi:10.1098/rspb.1998.0358. PMC 1689045. PMID 9628035.
- "Breaking the cycle of malaria transmission | NSF - National Science Foundation". www.nsf.gov. Retrieved 2017-09-16.
- Colpitts, Tonya M.; Conway, Michael J.; Montgomery, Ruth R.; Fikrig, Erol (2012-10-01). "West Nile Virus: Biology, Transmission, and Human Infection". Clinical Microbiology Reviews. 25 (4): 635–648. doi:10.1128/CMR.00045-12. ISSN 0893-8512. PMC 3485754. PMID 23034323.
- "WHO | Hepatitis C". WHO. Retrieved 20 August 2019.
- http://www.usa-journals.com/wp-content/uploads/2014/09/Tarish_Vol210.pdf
- De Filette, Marina; Ulbert, Sebastian; Diamond, Mike; Sanders, Niek N (2012). "Recent progress in West Nile virus diagnosis and vaccination". Veterinary Research. 43 (1): 16. doi:10.1186/1297-9716-43-16. ISSN 0928-4249. PMC 3311072. PMID 22380523.
- "Mosquito bites Symptoms - Mayo Clinic". www.mayoclinic.org. Retrieved 2017-10-01.
- "Dengue fever - Diagnosis". Mayo Clinic. Retrieved 2017-10-01.
- "West Nile virus". World Health Organization. Retrieved 2017-10-01.
- "West Nile virus - Diagnosis". Mayo Clinic. Retrieved 2017-10-01.
- "Testing for Zika Virus". CDC. 2017-08-30. Retrieved 2017-10-01.
- "Chikungunya". World Health Organization. Retrieved 2017-10-01.
- Gould, Ernest; Pettersson, John; Higgs, Stephen; Charrel, Remi; Lamballerie, Xavier de (2017). "Emerging arboviruses: Why today?". One Health. 4: 1–13. doi:10.1016/j.onehlt.2017.06.001. PMC 5501887. PMID 28785601.
- Strode, Clare; Donegan, Sarah; Garner, Paul; Enayati, Ahmad Ali; Hemingway, Janet (2014-03-18). "The Impact of Pyrethroid Resistance on the Efficacy of Insecticide-Treated Bed Nets against African Anopheline Mosquitoes: Systematic Review and Meta-Analysis". PLOS Medicine. 11 (3): e1001619. doi:10.1371/journal.pmed.1001619. ISSN 1549-1676. PMC 3958359. PMID 24642791.
- Achee, Nicole L.; Gould, Fred; Perkins, T. Alex; Jr, Robert C. Reiner; Morrison, Amy C.; Ritchie, Scott A.; Gubler, Duane J.; Teyssou, Remy; Scott, Thomas W. (2015-05-07). "A Critical Assessment of Vector Control for Dengue Prevention". PLOS Neglected Tropical Diseases. 9 (5): e0003655. doi:10.1371/journal.pntd.0003655. ISSN 1935-2735. PMC 4423954. PMID 25951103.
- Bellini, Romeo; Zeller, Herve; Van Bortel, Wim (2014-07-11). "A review of the vector management methods to prevent and control outbreaks of West Nile virus infection and the challenge for Europe". Parasites & Vectors. 7: 323. doi:10.1186/1756-3305-7-323. ISSN 1756-3305. PMC 4230500. PMID 25015004.
- "Yellow Fever Fact Sheet". World Health Organization. May 2016.
- "NIH begins study of vaccine to protect against mosquito-borne diseases". National Institutes of Health (NIH). 2017-02-21. Retrieved 2018-02-15.
- Choo, Monica Seungah; Blackwood, R. Alexander (2017-05-31). "School-based health education in Yucatan, Mexico about the Chikungunya virus and mosquito illness prevention". Infectious Disease Reports. 9 (2): 6894. doi:10.4081/idr.2017.6894. ISSN 2036-7449. PMC 5472339. PMID 28626536.
- Staples, J. Erin; Gershman, Mark; Fischer, Marc (2010). "Yellow Fever Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR. Recommendations and Reports : Morbidity and Mortality Weekly Report. Recommendations and Reports. 59 (RR-7): 1–27. PMID 20671663. Retrieved September 15, 2017.
- Cucunawangsih; Lugito, Nata Pratama Hardjo (March 15, 2017). "Trends of Dengue Disease Epidemiology". Virology:Research and Treatment. 8: 1178122X17695836. doi:10.1177/1178122X17695836. PMC 5428083. PMID 28579763.
- Sharma, Anshika; Lal, Sunil K (February 3, 2017). "Zika Virus: Transmission, Detection, Control, and Prevention". Front Microbiol. 8: 110. doi:10.3389/fmicb.2017.00110. PMC 5290000. PMID 28217114.
- Goupil, Brad A; Mores, Christopher N (November 30, 2016). "A Review of Chikungunya Virus-induced Arthralgia: Clinical Manifestations, Therapeutics, and Pathogenesis". Open Rheumatol J. 10: 129–140. doi:10.2174/1874312901610010129. PMC 5204064. PMID 28077980.
- content
- "Nuclear Energy to Cure Mosquitos Spread Diseases - ABC Live". abclive.in. 2016-09-06.
- Marreiros, Humberto; Marreiros, Humberto Filipe; Loff, Clara; Calado, Eulalia (January 2012). "Osteoporosis in paediatric patients with spina bifida". The Journal of Spinal Cord Medicine. 35 (1): 9–21. doi:10.1179/2045772311Y.0000000042. ISSN 1079-0268. PMC 3240921. PMID 22330186.
- Kuno, G; Gubler, D.J; Oliver, A (1993-01-01). "Use of 'original antigenic sin' theory to determine the serotypes of previous dengue infections". Transactions of the Royal Society of Tropical Medicine and Hygiene. 87 (1): 103–105. doi:10.1016/0035-9203(93)90444-u. ISSN 0035-9203. PMID 8465377.
- Halstead, Scott B. (June 1990). "Dengue and dengue hemorrhagic fever". Current Opinion in Infectious Diseases. 3 (3): 434–438. doi:10.1097/00001432-199006000-00020. ISSN 0951-7375.
- Rigau-Pérez, José G.; Clark, Gary G.; Gubler, Duane J.; Reiter, Paul; Sanders, Eduard J.; Vorndam, A. Vance (1998-09-19). "Dengue and dengue haemorrhagic fever". The Lancet. 352 (9132): 971–977. doi:10.1016/S0140-6736(97)12483-7. ISSN 0140-6736. PMID 9752834.
- G.D., Shanks; K., Biomndo; S.I., Hay; R.W., Snow (2000-05-01). "Changing patterns of clinical malaria since 1965 among a tea estate population located in the Kenyan highlands". Transactions of the Royal Society of Tropical Medicine and Hygiene. 94 (3): 253–255. doi:10.1016/S0035-9203(00)90310-9. ISSN 0035-9203. PMC 3272391. PMID 10974991.
- Lindsay, S. W.; Birley, M. H. (December 1996). "Climate change and malaria transmission". Annals of Tropical Medicine and Parasitology. 90 (6): 573–588. doi:10.1080/00034983.1996.11813087. ISSN 0003-4983. PMID 9039269.
- Glaser, Robert L.; Meola, Mark A. (2010-08-05). "The Native Wolbachia Endosymbionts of Drosophila melanogaster and Culex quinquefasciatus Increase Host Resistance to West Nile Virus Infection". PLOS One. 5 (8): e11977. doi:10.1371/journal.pone.0011977. ISSN 1932-6203. PMC 2916829. PMID 20700535.
- Hoffmann, A. A.; Montgomery, B. L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P. H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y. S. (2011). "Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission". Nature. 476 (7361): 454–457. doi:10.1038/nature10356. ISSN 1476-4687. PMID 21866160.
- Hoffmann, Ary A.; Iturbe-Ormaetxe, Inaki; Callahan, Ashley G.; Phillips, Ben L.; Billington, Katrina; Axford, Jason K.; Montgomery, Brian; Turley, Andrew P.; O'Neill, Scott L. (2014-09-11). "Stability of the wMel Wolbachia Infection following Invasion into Aedes aegypti Populations". PLOS Neglected Tropical Diseases. 8 (9): e3115. doi:10.1371/journal.pntd.0003115. ISSN 1935-2735. PMC 4161343. PMID 25211492.
- Schmidt, Tom L.; Barton, Nicholas H.; Rašić, Gordana; Turley, Andrew P.; Montgomery, Brian L.; Iturbe-Ormaetxe, Inaki; Cook, Peter E.; Ryan, Peter A.; Ritchie, Scott A. (2017-05-30). "Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti". PLOS Biology. 15 (5): e2001894. doi:10.1371/journal.pbio.2001894. ISSN 1545-7885. PMC 5448718. PMID 28557993.
- "Our research | World Mosquito Program". www.eliminatedengue.com. Retrieved 2018-02-14.