Acute myeloid leukemia
Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere with normal blood cell production.[1] Symptoms may include feeling tired, shortness of breath, easy bruising and bleeding, and increased risk of infection.[1] Occasionally, spread may occur to the brain, skin, or gums.[1] As an acute leukemia, AML progresses rapidly and is typically fatal within weeks or months if left untreated.[1][6]
| Acute myeloid leukemia | |
|---|---|
| Other names | Acute myelogenous leukemia, acute nonlymphocytic leukemia (ANLL), acute myeloblastic leukemia, acute granulocytic leukemia[1] |
| Bone marrow aspirate showing acute myeloid leukemia, arrows indicate Auer rods | |
| Specialty | Hematology, oncology |
| Symptoms | Feeling tired, shortness of breath, easy bruising and bleeding, increased risk of infection[1] |
| Usual onset | All ages, most frequently ~65–75 years old[2] |
| Risk factors | Smoking, previous chemotherapy or radiation therapy, myelodysplastic syndrome, benzene[1] |
| Diagnostic method | Bone marrow aspiration, blood test[3] |
| Treatment | Chemotherapy, radiation therapy, stem cell transplant[1][3] |
| Prognosis | Five-year survival ~27% (US)[2] |
| Frequency | 1 million (2015)[4] |
| Deaths | 147,100 (2015)[5] |
Risk factors include smoking, previous chemotherapy or radiation therapy, myelodysplastic syndrome, and exposure to the chemical benzene.[1] The underlying mechanism involves replacement of normal bone marrow with leukemia cells, which results in a drop in red blood cells, platelets, and normal white blood cells.[1] Diagnosis is generally based on bone marrow aspiration and specific blood tests.[3] AML has several subtypes for which treatments and outcomes may vary.[1]
AML typically is initially treated with chemotherapy, with the aim of inducing remission.[1] People may then go on to receive additional chemotherapy, radiation therapy, or a stem cell transplant.[1][3] The specific genetic mutations present within the cancer cells may guide therapy, as well as determine how long that person is likely to survive.[3]
In 2015, AML affected about one million people and resulted in 147,000 deaths globally.[4][5] It most commonly occurs in older adults.[2] Males are affected more often than females.[2] Five-year survival rate are about 35% in people under 60 years old and 10% in people over 60 years old.[3] Older people whose health is too poor for intensive chemotherapy have a typical survival of 5–10 months.[3] It accounts for roughly 1.8% of cancer deaths in the United States.[2]
Signs and symptoms
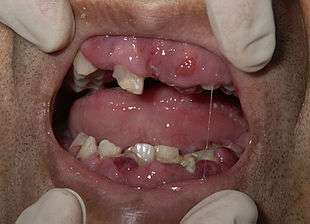
Most signs and symptoms of AML are caused by the replacement of normal blood cells with leukemic cells. A lack of normal white blood cell production makes people more susceptible to infections; while the leukemic cells themselves are derived from white blood cell precursors, they have no infection-fighting capacity.[7] A drop in red blood cell count (anemia) can cause fatigue, paleness, and shortness of breath. A lack of platelets can lead to easy bruising or bleeding with minor trauma.
The early signs of AML are often vague and nonspecific, and may be similar to those of influenza or other common illnesses. Some generalized symptoms include fever, fatigue, weight loss or loss of appetite, shortness of breath, anemia, easy bruising or bleeding, petechiae (flat, pin-head sized spots under the skin caused by bleeding), bone and joint pain, and persistent or frequent infections.[7]
Enlargement of the spleen may occur in AML, but it is typically mild and asymptomatic. Lymph node swelling is rare in AML, in contrast to acute lymphoblastic leukemia. The skin is involved about 10% of the time in the form of leukemia cutis. Rarely, Sweet's syndrome, a paraneoplastic inflammation of the skin, can occur with AML.[7]
Some people with AML may experience swelling of the gums because of infiltration of leukemic cells into the gum tissue. Rarely, the first sign of leukemia may be the development of a solid leukemic mass or tumor outside of the bone marrow, called a chloroma. Occasionally, a person may show no symptoms, and the leukemia may be discovered incidentally during a routine blood test.[8]
Risk factors
A number of risk factors for developing AML have been identified, including: other blood disorders, chemical exposures, ionizing radiation, and genetics.
Other blood disorders
"Preleukemic" blood disorders, such as myelodysplastic syndrome (MDS) or myeloproliferative neoplasms (MPN), can evolve into AML; the exact risk depends on the type of MDS/MPN.[9] The presence of asymptomatic clonal hematopoiesis also raises the risk of transformation into AML to 0.5–1.0% per year.[10]
Chemical exposure
Exposure to anticancer chemotherapy, in particular alkylating agents, can increase the risk of subsequently developing AML. The risk is highest about three to five years after chemotherapy.[11] Other chemotherapy agents, specifically epipodophyllotoxins and anthracyclines, have also been associated with treatment-related leukemias, which are often associated with specific chromosomal abnormalities in the leukemic cells.[12]
Occupational chemical exposure to benzene and other aromatic organic solvents is controversial as a cause of AML. Benzene and many of its derivatives are known to be carcinogenic in vitro. While some studies have suggested a link between occupational exposure to benzene and increased risk of AML,[13] others have suggested the attributable risk, if any, is slight.[14]
Radiation
High amounts of ionizing radiation exposure can increase the risk of AML. Survivors of the atomic bombings of Hiroshima and Nagasaki had an increased rate of AML,[15] as did radiologists exposed to high levels of X-rays prior to the adoption of modern radiation safety practices.[16] People treated with ionizing radiation after treatment for prostate cancer, non-Hodgkin lymphoma, lung cancer, and breast cancer have the highest chance of acquiring AML, but this increased risk returns to the background risk observed in the general population after 12 years.[17]
Genetics
A hereditary risk for AML appears to exist. Multiple cases of AML developing in a family at a rate higher than predicted by chance alone have been reported.[18][19][20][21] Several congenital conditions may increase the risk of leukemia; the most common is probably Down syndrome, which is associated with a 10- to 18-fold increase in the risk of AML.[22] In a second example, inactivating mutations in one of the two parental GATA2 genes lead to a reduction, i.e. a haploinsufficiency, in the cellular levels of the gene's product, the GATA2 transcription factor, and thereby to a rare autosomal dominant genetic disease, GATA2 deficiency. This disease is associated with a highly variable set of disorders including an exceedingly high risk of developing AML.[23][24] The specific genetic abnormalities causing AML usually vary between those who develop the disease as a child versus an adult.[25] However, GATA2 deficiency-induced AML may first appear in children or adults.[24]
Diagnosis
The first clue to a diagnosis of AML is typically an abnormal result on a complete blood count. While an excess of abnormal white blood cells (leukocytosis) is a common finding with the leukemia, and leukemic blasts are sometimes seen, AML can also present with isolated decreases in platelets, red blood cells, or even with a low white blood cell count (leukopenia).[26] While a presumptive diagnosis of AML can be made by examination of the peripheral blood smear when there are circulating leukemic blasts, a definitive diagnosis usually requires an adequate bone marrow aspiration and biopsy as well as ruling out pernicious anemia (Vitamin B12 deficiency), folic acid deficiency and copper deficiency.[27][28][29][30]
Marrow or blood is examined under light microscopy, as well as flow cytometry, to diagnose the presence of leukemia, to differentiate AML from other types of leukemia (e.g. acute lymphoblastic leukemia – ALL), and to classify the subtype of disease. A sample of marrow or blood is typically also tested for chromosomal abnormalities by routine cytogenetics or fluorescent in situ hybridization. Genetic studies may also be performed to look for specific mutations in genes such as FLT3, nucleophosmin, and KIT, which may influence the outcome of the disease.[31]
Cytochemical stains on blood and bone marrow smears are helpful in the distinction of AML from ALL, and in subclassification of AML. The combination of a myeloperoxidase or Sudan black stain and a nonspecific esterase stain will provide the desired information in most cases. The myeloperoxidase or Sudan black reactions are most useful in establishing the identity of AML and distinguishing it from ALL. The nonspecific esterase stain is used to identify a monocytic component in AMLs and to distinguish a poorly differentiated monoblastic leukemia from ALL.[32]
The diagnosis and classification of AML can be challenging, and should be performed by a qualified hematopathologist or hematologist. In straightforward cases, the presence of certain morphologic features (such as Auer rods) or specific flow cytometry results can distinguish AML from other leukemias; however, in the absence of such features, diagnosis may be more difficult.[33]
The two most commonly used classification schemata for AML are the older French-American-British (FAB) system and the newer World Health Organization (WHO) system. According to the widely used WHO criteria, the diagnosis of AML is established by demonstrating involvement of more than 20% of the blood and/or bone marrow by leukemic myeloblasts, except in the three best prognosis forms of acute myeloid leukemia with recurrent genetic abnormalities (t(8;21), inv(16), and t(15;17)) in which the presence of the genetic abnormality is diagnostic irrespective of blast percent.[34][35] The FAB classification is a bit more stringent, requiring a blast percentage of at least 30% in bone marrow or peripheral blood for the diagnosis of AML.[36] AML must be carefully differentiated from "preleukemic" conditions such as myelodysplastic or myeloproliferative syndromes, which are treated differently.
Because acute promyelocytic leukemia (APL) has the highest curability and requires a unique form of treatment, it is important to quickly establish or exclude the diagnosis of this subtype of leukemia. Fluorescent in situ hybridization performed on blood or bone marrow is often used for this purpose, as it readily identifies the chromosomal translocation [t(15;17)(q22;q12);] that characterizes APL. There is also a need to molecularly detect the presence of PML/RARA fusion protein, which is an oncogenic product of that translocation.[37]
World Health Organization
The WHO 2008 classification of AML attempts to be more clinically useful and to produce more meaningful prognostic information than the FAB criteria. Each of the WHO categories contains numerous descriptive subcategories of interest to the hematopathologist and oncologist; however, most of the clinically significant information in the WHO schema is communicated via categorization into one of the subtypes listed below.
The WHO subtypes of AML are:[38]
| Name | Description | ICD-O |
|---|---|---|
| Acute myeloid leukemia with recurrent genetic abnormalities | Includes:
|
Multiple |
| AML with myelodysplasia-related changes | This category includes people who have had a prior documented myelodysplastic syndrome (MDS) or myeloproliferative disease (MPD) that then has transformed into AML, or who have cytogenetic abnormalities characteristic for this type of AML (with previous history of MDS or MPD that has gone unnoticed in the past, but the cytogenetics is still suggestive of MDS/MPD history). This category of AML occurs most often in elderly people and often has a worse prognosis. Includes:
|
M9895/3 |
| Therapy-related myeloid neoplasms | This category includes people who have had prior chemotherapy and/or radiation and subsequently develop AML or MDS. These leukemias may be characterized by specific chromosomal abnormalities, and often carry a worse prognosis. | M9920/3 |
| Myeloid sarcoma | This category includes myeloid sarcoma. | |
| Myeloid proliferations related to Down syndrome | This category includes so-called "transient abnormal myelopoiesis" and "myeloid leukemia associated with Down syndrome" | |
| Blastic plasmacytoid dendritic cell neoplasm | This category includes so-called "blastic plasmacytoid dendritic cell neoplasm" | |
| AML not otherwise categorized | Includes subtypes of AML that do not fall into the above categories
|
M9861/3 |
Acute leukemias of ambiguous lineage (also known as mixed phenotype or biphenotypic acute leukemia) occur when the leukemic cells can not be classified as either myeloid or lymphoid cells, or where both types of cells are present.
French-American-British
The French-American-British (FAB) classification system divides AML into eight subtypes, M0 through to M7, based on the type of cell from which the leukemia developed and its degree of maturity. This is done by examining the appearance of the malignant cells with light microscopy and/or by using cytogenetics to characterize any underlying chromosomal abnormalities. The subtypes have varying prognoses and responses to therapy. Although the WHO classification (see above) may be more useful, the FAB system is still widely used.
Six FAB subtypes (M1 through to M6) were initially proposed in 1976,[39] although later revisions added M7 in 1985[40] and M0 in 1987.[41]
| Type | Name | Cytogenetics | Percentage of adults with AML | Immunophenotype[42] | ||||
|---|---|---|---|---|---|---|---|---|
| CD14 | CD15 | CD33 | HLA-DR | Other | ||||
| M0 | acute myeloblastic leukemia, minimally differentiated | 5%[43] | − [44] | − [44] | + [44] | + [44] | MPO − [45] | |
| M1 | acute myeloblastic leukemia, without maturation | 15%[43] | − | − | + | + | MPO + [45] | |
| M2 | acute myeloblastic leukemia, with granulocytic maturation | t(8;21)(q22;q22), t(6;9) | 25%[43] | − | + | + | + | |
| M3 | promyelocytic, or acute promyelocytic leukemia (APL) | t(15;17) | 10%[43] | − | + | + | − | |
| M4 | acute myelomonocytic leukemia | inv(16)(p13q22), del(16q) | 20%[43] | <45% | + | + | + | |
| M4eo | myelomonocytic together with bone marrow eosinophilia | inv(16), t(16;16) | 5%[43] | +/− [46] | + [47] | + [47] | CD2+ [47] | |
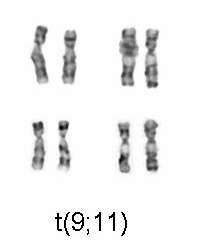
| M5 | acute monoblastic leukemia (M5a) or acute monocytic leukemia (M5b) | del (11q), t(9;11), t(11;19) | 10%[43] | >55% | + | + | + | |
| M6 | acute erythroid leukemias, including erythroleukemia (M6a) and very rare pure erythroid leukemia (M6b) | 5%[43] | − | +/− | +/− | +/− | Glycophorin + | |
| M7 | acute megakaryoblastic leukemia | t(1;22) | 5%[43] | − | − | + | +/− | CD41/CD61+ |
The morphologic subtypes of AML also include rare types not included in the FAB system, such as acute basophilic leukemia, which was proposed as a ninth subtype, M8, in 1999.[48]
Pathophysiology
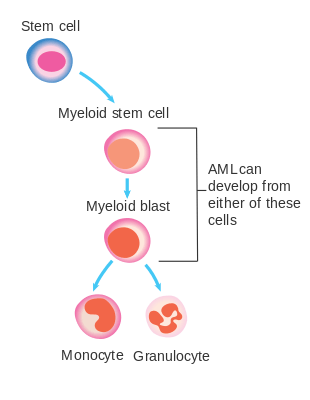
The malignant cell in AML is the myeloblast. In normal hematopoiesis, the myeloblast is an immature precursor of myeloid white blood cells; a normal myeloblast will gradually mature into a mature white blood cell. In AML, though, a single myeloblast accumulates genetic changes which "freeze" the cell in its immature state and prevent differentiation.[49] Such a mutation alone does not cause leukemia; however, when such a "differentiation arrest" is combined with other mutations which disrupt genes controlling proliferation, the result is the uncontrolled growth of an immature clone of cells, leading to the clinical entity of AML.[50]
Much of the diversity and heterogeneity of AML is because leukemic transformation can occur at a number of different steps along the differentiation pathway.[51] Modern classification schemes for AML recognize that the characteristics and behavior of the leukemic cell (and the leukemia) may depend on the stage at which differentiation was halted.
Specific cytogenetic abnormalities can be found in many people with AML; the types of chromosomal abnormalities often have prognostic significance.[52] The chromosomal translocations encode abnormal fusion proteins, usually transcription factors whose altered properties may cause the "differentiation arrest".[53] For example, in APL, the t(15;17) translocation produces a PML-RARA fusion protein which binds to the retinoic acid receptor element in the promoters of several myeloid-specific genes and inhibits myeloid differentiation.[54]
The clinical signs and symptoms of AML result from the growth of leukemic clone cells, which tends to interfere with the development of normal blood cells in the bone marrow.[55] This leads to neutropenia, anemia, and thrombocytopenia. The symptoms of AML are, in turn, often due to the low numbers of these normal blood elements. In rare cases, people with AML can develop a chloroma, or solid tumor of leukemic cells outside the bone marrow, which can cause various symptoms depending on its location.[7]
An important pathophysiological mechanism of leukemogenesis in AML is the epigenetic induction of dedifferentiation by genetic mutations that alter the function of epigenetic enzymes, such as the DNA demethylase TET2 and the metabolic enzymes IDH1 and IDH2,[56] which lead to the generation of a novel oncometabolite, D-2-hydroxyglutarate, which inhibits the activity of epigenetic enzymes such as TET2.[57] The hypothesis is that such epigenetic mutations lead to the silencing of tumor suppressor genes and/or the activation of proto-oncogenes.[58]
Treatment
First-line treatment of AML consists primarily of chemotherapy, and is divided into two phases: induction and postremission (or consolidation) therapy. The goal of induction therapy is to achieve a complete remission by reducing the number of leukemic cells to an undetectable level; the goal of consolidation therapy is to eliminate any residual undetectable disease and achieve a cure.[59] Hematopoietic stem cell transplantation is usually considered if induction chemotherapy fails or after a person relapses, although transplantation is also sometimes used as front-line therapy for people with high-risk disease. Efforts to use tyrosine kinase inhibitors in AML continue.[60]
Induction
All FAB subtypes except M3 are usually given induction chemotherapy with cytarabine (ara-C) and an anthracycline (most often daunorubicin).[61] This induction chemotherapy regimen is known as "7+3" (or "3+7"), because the cytarabine is given as a continuous IV infusion for seven consecutive days while the anthracycline is given for three consecutive days as an IV push. Up to 70% of people with AML will achieve a remission with this protocol.[62] Other alternative induction regimens, including high-dose cytarabine alone, FLAG-like regimens or investigational agents, may also be used.[63][64] Because of the toxic effects of therapy, including myelosuppression and an increased risk of infection, induction chemotherapy may not be offered to the very elderly, and the options may include less intense chemotherapy or palliative care.
The M3 subtype of AML, also known as acute promyelocytic leukemia (APL), is treated with either arsenic trioxide (ATO) monotherapy,[65][66] or the drug all-trans-retinoic acid (ATRA) in addition to induction chemotherapy, usually an anthracycline.[67][68][69] Care must be taken to prevent disseminated intravascular coagulation (DIC), complicating the treatment of APL when the promyelocytes release the contents of their granules into the peripheral circulation. APL is eminently curable, with well-documented treatment protocols.
The goal of the induction phase is to reach a complete remission. Complete remission does not mean the disease has been cured; rather, it signifies no disease can be detected with available diagnostic methods.[61] Complete remission is obtained in about 50%–75% of newly diagnosed adults, although this may vary based on the prognostic factors described above.[70] The length of remission depends on the prognostic features of the original leukemia. In general, all remissions will fail without additional consolidation therapy.[71]
There is insufficient evidence to determine if prescribing all-trans retinoic acid (ATRA) in addition to chemotherapy to adults that suffer from acute myeloid leukaemia is helpful.[72]
Consolidation
Even after complete remission is achieved, leukemic cells likely remain in numbers too small to be detected with current diagnostic techniques. If no further postremission or consolidation therapy is given, almost all people with AML will eventually relapse.[71] Therefore, more therapy is necessary to eliminate nondetectable disease and prevent relapse – that is, to achieve a cure.
The specific type of postremission therapy is individualized based on a person's prognostic factors (see above) and general health. For good-prognosis leukemias (i.e. inv(16), t(8;21), and t(15;17)), people will typically undergo an additional three to five courses of intensive chemotherapy, known as consolidation chemotherapy.[73][74] For people at high risk of relapse (e.g. those with high-risk cytogenetics, underlying MDS, or therapy-related AML), allogeneic stem cell transplantation is usually recommended if the person is able to tolerate a transplant and has a suitable donor. The best postremission therapy for intermediate-risk AML (normal cytogenetics or cytogenetic changes not falling into good-risk or high-risk groups) is less clear and depends on the specific situation, including the age and overall health of the person, the person's values, and whether a suitable stem cell donor is available.[74]
For people who are not eligible for a stem cell transplant, immunotherapy with a combination of histamine dihydrochloride (Ceplene) and interleukin 2 (Proleukin) after the completion of consolidation has been shown to reduce the absolute relapse risk by 14%, translating to a 50% increase in the likelihood of maintained remission.[75]
Relapsed AML
For people with relapsed AML, the only proven potentially curative therapy is a hematopoietic stem cell transplant, if one has not already been performed.[76][77][78] In 2000, the monoclonal antibody-linked cytotoxic agent gemtuzumab ozogamicin (Mylotarg) was approved in the United States for people aged more than 60 years with relapsed AML who are not candidates for high-dose chemotherapy.[79] This drug was voluntarily withdrawn from the market by its manufacturer, Pfizer in 2010, but newer data aided its reintroduction in 2017.[80][81]
Since treatment options for relapsed AML are so limited, palliative care or enrollment in a clinical trial may be offered.
Supportive treatment
Adding aerobic physical exercises to the standard of care may result in little to no difference in the mortality, in the quality of life and in the physical functioning.[82]
Side effects
Treatments for AML like chemotherapy or stem cell transplant can trigger side effects. People that receive a stem cell transplant are at risk for developing a graft-versus-host disease,[83] and suffer from bleeding events that may require platelet transfusions.[84][85]
Prognosis
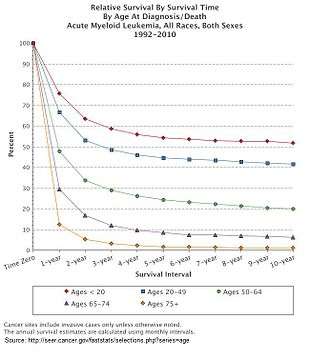
AML is a curable disease. The chance of cure for a specific person depends on a number of prognostic factors.[86]
Cytogenetics
The single most important prognostic factor in AML is cytogenetics, or the chromosomal structure of the leukemic cell. Certain cytogenetic abnormalities are associated with very good outcomes (for example, the (15;17) translocation in APL). About half of people with AML have "normal" cytogenetics; they fall into an intermediate risk group. A number of other cytogenetic abnormalities are known to associate with a poor prognosis and a high risk of relapse after treatment.[87][88][89]
The first publication to address cytogenetics and prognosis was the MRC trial of 1998:[90]
| Risk Category | Abnormality | Five-year survival | Relapse rate |
|---|---|---|---|
| Good | t(8;21), t(15;17), inv(16) | 70% | 33% |
| Intermediate | Normal, +8, +21, +22, del(7q), del(9q), Abnormal 11q23, all other structural or numerical changes | 48% | 50% |
| Poor | −5, −7, del(5q), Abnormal 3q, Complex cytogenetics | 15% | 78% |
Later, the Southwest Oncology Group and Eastern Cooperative Oncology Group[91] and, later still, Cancer and Leukemia Group B published other, mostly overlapping lists of cytogenetics prognostication in leukemia.[89]
Myelodysplastic syndrome
AML arising from a pre-existing myelodysplastic syndrome (MDS) or myeloproliferative disease (so-called secondary AML) has a worse prognosis, as does treatment-related AML arising after chemotherapy for another previous malignancy. Both of these entities are associated with a high rate of unfavorable cytogenetic abnormalities.[92][93][94]
Other prognostic markers
In some studies, age >60 years and elevated lactate dehydrogenase level were also associated with poorer outcomes.[95] As with most forms of cancer, performance status (i.e. the general physical condition and activity level of the person) plays a major role in prognosis as well.
The five-year survival rate is about 25% overall. Age plays a significant role: 40% of people under the age of 60, but just 10% of those over it, live five years after diagnosis.[96]
Genotype
A large number of molecular alterations are under study for their prognostic impact in AML. However, only FLT3-ITD, NPM1, CEBPA and c-KIT are currently included in validated international risk stratification schema. These are expected to increase rapidly in the near future.[3] FLT3 internal tandem duplications (ITDs) have been shown to confer a poorer prognosis in AML with normal cytogenetics. Several FLT3 inhibitors have undergone clinical trials, with mixed results. Two other mutations – NPM1 and biallelic CEBPA are associated with improved outcomes, especially in people with normal cytogenetics and are used in current risk stratification algorithms.[3]
Researchers are investigating the clinical significance of c-KIT mutations in AML. These are prevalent, and potentially clinically relevant because of the availability of tyrosine kinase inhibitors, such as imatinib and sunitinib that can block the activity of c-KIT pharmacologically.[3] It is expected that additional markers (e.g., RUNX1, ASXL1, and TP53) that have consistently been associated with an inferior outcome will soon be included in these recommendations. The prognostic importance of other mutated genes (e.g., DNMT3A, IDH1, IDH2) is less clear.[3][56]
Expectation of cure
Cure rates in clinical trials have ranged from 20–45%;[97][98] although clinical trials often include only younger people and those able to tolerate aggressive therapies. The overall cure rate for all people with AML (including the elderly and those unable to tolerate aggressive therapy) is likely lower. Cure rates for APL can be as high as 98%.[99]
Relapse
Relapse is common, and the prognosis is poor.[96] Long-term survival after a relapse is so rare that the only known case was submitted to the Catholic Church as evidence of a miracle attributed to Marie-Marguerite d'Youville.[100]
Epidemiology
AML is a relatively rare cancer. There are approximately 10,500 new cases each year in the United States, and the incidence rate has remained stable from 1995 through 2005. AML accounts for 1.2% of all cancer deaths in the United States.[101]
The incidence of AML increases with age; the median age at diagnosis is 63 years. AML accounts for about 90% of all acute leukemias in adults, but is rare in children.[101] The rate of therapy-related AML (that is, AML caused by previous chemotherapy) is rising; therapy-related disease currently accounts for about 10–20% of all cases of AML.[102] AML is slightly more common in men, with a male-to-female ratio of 1.3:1.[103]
There is some geographic variation in the incidence of AML. In adults, the highest rates are seen in North America, Europe, and Oceania, while adult AML is rarer in Asia and Latin America.[104][105] In contrast, childhood AML is less common in North America and India than in other parts of Asia.[106] These differences may be due to population genetics, environmental factors, or a combination of the two.
AML accounts for 34% of all leukemia cases in the UK, and around 2,900 people were diagnosed with the disease in 2011.[107]
History
The first published description of a case of leukemia in medical literature dates to 1827 when French physician Alfred-Armand-Louis-Marie Velpeau described a 63-year-old florist who developed an illness characterized by fever, weakness, urinary stones, and substantial enlargement of the liver and spleen. Velpeau noted the blood of this person had a consistency "like gruel", and speculated the appearance of the blood was due to white corpuscles.[7]:1071 In 1845, a series of people who died with enlarged spleens and changes in the "colors and consistencies of their blood" was reported by the Edinburgh-based pathologist J.H. Bennett; he used the term "leucocythemia" to describe this pathological condition.[108]
The term "leukemia" was coined by Rudolf Virchow, the renowned German pathologist, in 1856. As a pioneer in the use of the light microscope in pathology, Virchow was the first to describe the abnormal excess of white blood cells in people with the clinical syndrome described by Velpeau and Bennett. As Virchow was uncertain of the etiology of the white blood cell excess, he used the purely descriptive term "leukemia" (Greek: "white blood") to refer to the condition.[109]
Further advances in the understanding of AML occurred rapidly with the development of new technology. In 1877, Paul Ehrlich developed a technique of staining blood films which allowed him to describe in detail normal and abnormal white blood cells. Wilhelm Ebstein introduced the term "acute leukemia" in 1889 to differentiate rapidly progressive and fatal leukemias from the more indolent chronic leukemias.[110] The term "myeloid" was coined by Franz Ernst Christian Neumann in 1869, as he was the first to recognize white blood cells were made in the bone marrow (Greek: μυєλός, myelos, lit. '(bone) marrow') as opposed to the spleen. The technique of bone marrow examination to diagnose leukemia was first described in 1879 by Mosler.[111] Finally, in 1900, the myeloblast, which is the malignant cell in AML, was characterized by Otto Naegeli, who divided the leukemias into myeloid and lymphocytic.[112][113]
In 2008, AML became the first cancer genome to be fully sequenced. DNA extracted from leukemic cells were compared to unaffected skin.[114] The leukemic cells contained acquired mutations in several genes that had not previously been associated with the disease.
Pregnancy
Leukemia is rarely associated with pregnancy, affecting only about 1 in 10,000 pregnant women.[115] How it is handled depends primarily on the type of leukemia. Acute leukemias normally require prompt, aggressive treatment, despite significant risks of pregnancy loss and birth defects, especially if chemotherapy is given during the developmentally sensitive first trimester.[115]
References
- "Adult Acute Myeloid Leukemia Treatment". National Cancer Institute. 6 March 2017. Retrieved 19 December 2017.
- "Acute Myeloid Leukemia – Cancer Stat Facts". NCI. Retrieved 10 May 2017.
- Döhner H, Weisdorf DJ, Bloomfield CD (September 2015). "Acute Myeloid Leukemia". The New England Journal of Medicine. 373 (12): 1136–52. doi:10.1056/NEJMra1406184. PMID 26376137. S2CID 40314260.
- "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. October 2016. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. October 2016. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- Marino BS, Fine KS (2013). Blueprints Pediatrics. Lippincott Williams & Wilkins. p. 205. ISBN 9781451116045.
- Hoffman R (2005). Hematology: Basic Principles and Practice (4th ed.). St. Louis, Mo.: Elsevier Churchill Livingstone. pp. 1074–75. ISBN 978-0-443-06629-0.
- Abeloff M (2004). Clinical Oncology (3rd ed.). St. Louis, Mo.: Elsevier Churchill Livingstone. p. 2834. ISBN 978-0-443-06629-0.
- Sanz GF, Sanz MA, Vallespí T, Cañizo MC, Torrabadella M, García S, et al. (July 1989). "Two regression models and a scoring system for predicting survival and planning treatment in myelodysplastic syndromes: a multivariate analysis of prognostic factors in 370 patients". Blood. 74 (1): 395–408. doi:10.1182/blood.V74.1.395.395. PMID 2752119.
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. (December 2014). "Age-related clonal hematopoiesis associated with adverse outcomes". The New England Journal of Medicine. 371 (26): 2488–98. doi:10.1056/NEJMoa1408617. PMC 4306669. PMID 25426837.
- Le Beau MM, Albain KS, Larson RA, Vardiman JW, Davis EM, Blough RR, et al. (March 1986). "Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no. 5 and 7". Journal of Clinical Oncology. 4 (3): 325–45. doi:10.1200/JCO.1986.4.3.325. PMID 3950675.
- Thirman MJ, Gill HJ, Burnett RC, Mbangkollo D, McCabe NR, Kobayashi H, et al. (September 1993). "Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations". The New England Journal of Medicine. 329 (13): 909–14. doi:10.1056/NEJM199309233291302. PMID 8361504.
- Austin H, Delzell E, Cole P (March 1988). "Benzene and leukemia. A review of the literature and a risk assessment". American Journal of Epidemiology. 127 (3): 419–39. doi:10.1093/oxfordjournals.aje.a114820. PMID 3277397.
- Linet MS (1985). The Leukemias: Epidemiologic Aspects. New York: Oxford University Press.
- Bizzozero OJ, Johnson KG, Ciocco A (May 1966). "Radiation-related leukemia in Hiroshima and Nagasaki, 1946-1964. I. Distribution, incidence and appearance time". The New England Journal of Medicine. 274 (20): 1095–101. doi:10.1056/NEJM196605192742001. PMID 5932020.
- Yoshinaga S, Mabuchi K, Sigurdson AJ, Doody MM, Ron E (November 2004). "Cancer risks among radiologists and radiologic technologists: review of epidemiologic studies". Radiology. 233 (2): 313–21. doi:10.1148/radiol.2332031119. PMID 15375227. S2CID 20643232.
- Radivoyevitch T, Sachs RK, Gale RP, Molenaar RJ, Brenner DJ, Hill BT, et al. (February 2016). "Defining AML and MDS second cancer risk dynamics after diagnoses of first cancers treated or not with radiation". Leukemia. 30 (2): 285–94. doi:10.1038/leu.2015.258. PMID 26460209.
- Taylor GM, Birch JM (1996). "The hereditary basis of human leukemia". In Henderson ES, Lister TA, Greaves MF (eds.). Leukemia (6th ed.). Philadelphia: WB Saunders. p. 210. ISBN 978-0-7216-5381-5.
- Horwitz M, Goode EL, Jarvik GP (November 1996). "Anticipation in familial leukemia". American Journal of Human Genetics. 59 (5): 990–8. PMC 1914843. PMID 8900225.
- Crittenden LB (June 1961). "An interpretation of familial aggregation based on multiple genetic and environmental factors". Annals of the New York Academy of Sciences. 91 (3): 769–80. Bibcode:1961NYASA..91..769C. doi:10.1111/j.1749-6632.1961.tb31106.x. PMID 13696504.
- Horwitz M (August 1997). "The genetics of familial leukemia". Leukemia. 11 (8): 1347–59. doi:10.1038/sj.leu.2400707. PMID 9264391.
- Evans DI, Steward JK (December 1972). "Down's syndrome and leukaemia". Lancet. 2 (7790): 1322. doi:10.1016/S0140-6736(72)92704-3. PMID 4117858.
- Crispino JD, Horwitz MS (April 2017). "GATA factor mutations in hematologic disease". Blood. 129 (15): 2103–2110. doi:10.1182/blood-2016-09-687889. PMC 5391620. PMID 28179280.
- Hirabayashi S, Wlodarski MW, Kozyra E, Niemeyer CM (August 2017). "Heterogeneity of GATA2-related myeloid neoplasms". International Journal of Hematology. 106 (2): 175–182. doi:10.1007/s12185-017-2285-2. PMID 28643018.
- Bolouri H, Farrar JE, Triche T, Ries RE, Lim EL, Alonzo TA, et al. (January 2018). "The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions". Nature Medicine. 24 (1): 103–112. doi:10.1038/nm.4439. PMC 5907936. PMID 29227476.
- Abeloff, Martin et al. (2004), p. 2834.
- Dokal IS, Cox TM, Galton DA (May 1990). "Vitamin B-12 and folate deficiency presenting as leukaemia". Bmj. 300 (6734): 1263–4. doi:10.1136/bmj.300.6734.1263. PMC 1662842. PMID 2354298.
- Aitelli C, Wasson L, Page R (March 2004). "Pernicious anemia: presentations mimicking acute leukemia". Southern Medical Journal. 97 (3): 295–7. doi:10.1097/01.SMJ.0000082003.98003.88. PMID 15043340.
- Zuo Z, Polski JM, Kasyan A, Medeiros LJ (September 2010). "Acute erythroid leukemia". Archives of Pathology & Laboratory Medicine. 134 (9): 1261–70. doi:10.1043/2009-0350-RA.1 (inactive 25 May 2020). PMID 20807044.
- Barzi A, Sekeres MA (January 2010). "Myelodysplastic syndromes: a practical approach to diagnosis and treatment". Cleveland Clinic Journal of Medicine. 77 (1): 37–44. doi:10.3949/ccjm.77a.09069. PMID 20048028. S2CID 207413787.
- Baldus CD, Mrózek K, Marcucci G, Bloomfield CD (June 2007). "Clinical outcome of de novo acute myeloid leukaemia patients with normal cytogenetics is affected by molecular genetic alterations: a concise review". British Journal of Haematology. 137 (5): 387–400. doi:10.1111/j.1365-2141.2007.06566.x. PMID 17488484.
- Vardiman JW, Harris NL, Brunning RD (October 2002). "The World Health Organization (WHO) classification of the myeloid neoplasms". Blood. 100 (7): 2292–302. doi:10.1182/blood-2002-04-1199. PMID 12239137. S2CID 9413654.
- Abeloff, Martin et al. (2004), p. 2835.
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. (December 1999). "The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997". Annals of Oncology. 10 (12): 1419–32. doi:10.1023/A:1008375931236. PMID 10643532.
- Foucar K. "Bone Marrow Pathology" (PDF) (3rd ed.). ASCP. Archived from the original (PDF) on 19 March 2013. Retrieved 18 March 2016.
- Amin HM, Yang Y, Shen Y, Estey EH, Giles FJ, Pierce SA, et al. (September 2005). "Having a higher blast percentage in circulation than bone marrow: clinical implications in myelodysplastic syndrome and acute lymphoid and myeloid leukemias". Leukemia. 19 (9): 1567–72. doi:10.1038/sj.leu.2403876. PMID 16049515.
- Grimwade D, Howe K, Langabeer S, Davies L, Oliver F, Walker H, et al. (September 1996). "Establishing the presence of the t(15;17) in suspected acute promyelocytic leukaemia: cytogenetic, molecular and PML immunofluorescence assessment of patients entered into the M.R.C. ATRA trial. M.R.C. Adult Leukaemia Working Party". British Journal of Haematology. 94 (3): 557–73. doi:10.1046/j.1365-2141.1996.d01-1004.x (inactive 25 May 2020). PMID 8790159.
- Falini B, Tiacci E, Martelli MP, Ascani S, Pileri SA (October 2010). "New classification of acute myeloid leukemia and precursor-related neoplasms: changes and unsolved issues". Discovery Medicine. 10 (53): 281–92. PMID 21034669.
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C (August 1976). "Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group". British Journal of Haematology. 33 (4): 451–8. doi:10.1111/j.1365-2141.1976.tb03563.x. PMID 188440.
- Bloomfield CD, Brunning RD (September 1985). "FAB M7: acute megakaryoblastic leukemia--beyond morphology". Annals of Internal Medicine. 103 (3): 450–2. doi:10.7326/0003-4819-103-3-450. PMID 4040724.
- Lee EJ, Pollak A, Leavitt RD, Testa JR, Schiffer CA (November 1987). "Minimally differentiated acute nonlymphocytic leukemia: a distinct entity". Blood. 70 (5): 1400–6. doi:10.1182/blood.v70.5.1400.bloodjournal7051400. PMID 3663939.
- Unless otherwise specified in boxes, reference is: Page 97 in: Sun T (2008). Flow cytometry and immunohistochemistry for hematologic neoplasms. Philadelphia: Lippincott Williams & Wilkins. ISBN 978-0-7817-8400-9. OCLC 85862340.
- Seiter K, Jules EH (20 May 2011). "Acute Myeloid Leukemia Staging". Retrieved 26 August 2011.
- Mihova D. "Leukemia acute - Acute myeloid leukemia with minimal differentiation (FAB AML M0)". Pathology Outlines. Topic Completed: 1 March 2013. Minor changes: 19 November 2019}}
- Salem DA, Abd El-Aziz SM (June 2012). "Flowcytometric immunophenotypic profile of acute leukemia: mansoura experience". Indian Journal of Hematology & Blood Transfusion. 28 (2): 89–96. doi:10.1007/s12288-011-0110-2. PMC 3332273. PMID 23730015.
- Partial expression: Adriaansen HJ, te Boekhorst PA, Hagemeijer AM, van der Schoot CE, Delwel HR, van Dongen JJ (June 1993). "Acute myeloid leukemia M4 with bone marrow eosinophilia (M4Eo) and inv(16)(p13q22) exhibits a specific immunophenotype with CD2 expression". Blood. 81 (11): 3043–51. doi:10.1182/blood.V81.11.3043.bloodjournal81113043. PMID 8098967.
- Page 99 in: Sun T (2009). Atlas of hematologic neoplasms. Dordrecht New York: Springer. ISBN 978-0-387-89848-3. OCLC 432709321.
- Duchayne E, Demur C, Rubie H, Robert A, Dastugue N (January 1999). "Diagnosis of acute basophilic leukemia". Leukemia & Lymphoma. 32 (3–4): 269–78. doi:10.3109/10428199909167387. PMID 10037024.
- Fialkow PJ (October 1976). "Clonal origin of human tumors". Biochimica et Biophysica Acta. 458 (3): 283–321. doi:10.1016/0304-419X(76)90003-2. PMID 1067873.
- Fialkow PJ, Janssen JW, Bartram CR (April 1991). "Clonal remissions in acute nonlymphocytic leukemia: evidence for a multistep pathogenesis of the malignancy". Blood. 77 (7): 1415–7. doi:10.1182/blood.V77.7.1415.1415. PMID 2009365.
- Bonnet D, Dick JE (July 1997). "Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell". Nature Medicine. 3 (7): 730–7. doi:10.1038/nm0797-730. PMID 9212098.
- Abeloff, Martin et al. (2004), pp. 2831–32.
- Greer JP, Foerster J, Lukens JN, Rogers GM, Paraskevas F, Glader BE, eds. (2004). Wintrobe's Clinical Hematology (11th ed.). Philadelphia: Lippincott, Williams, and Wilkins. pp. 2045–2062. ISBN 978-0-7817-3650-3.
- Melnick A, Licht JD (May 1999). "Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia". Blood. 93 (10): 3167–215. doi:10.1182/blood.V93.10.3167.410k44_3167_3215. PMID 10233871.
- Abeloff, Martin et al. (2004), p. 2828.
- Molenaar RJ, Thota S, Nagata Y, Patel B, Clemente M, Przychodzen B, et al. (November 2015). "Clinical and biological implications of ancestral and non-ancestral IDH1 and IDH2 mutations in myeloid neoplasms". Leukemia. 29 (11): 2134–42. doi:10.1038/leu.2015.91. PMC 5821256. PMID 25836588.
- Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, Bleeker FE (December 2014). "The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation". Biochimica et Biophysica Acta. 1846 (2): 326–41. doi:10.1016/j.bbcan.2014.05.004. PMID 24880135.
- Sharma S, Kelly TK, Jones PA (January 2010). "Epigenetics in cancer". Carcinogenesis. 31 (1): 27–36. doi:10.1093/carcin/bgp220. PMC 2802667. PMID 19752007.
- "Acute myeloid leukemia". The Mount Sinai Hospital. September 2011. Archived from the original on 7 August 2012.
- Kayser S, Levis MJ (February 2014). "FLT3 tyrosine kinase inhibitors in acute myeloid leukemia: clinical implications and limitations". Leukemia & Lymphoma. 55 (2): 243–55. doi:10.3109/10428194.2013.800198. PMC 4333682. PMID 23631653.
- Abeloff, Martin et al. (2004), pp. 2835–39.
- Bishop JF (February 1997). "The treatment of adult acute myeloid leukemia". Seminars in Oncology. 24 (1): 57–69. PMID 9045305.
- Weick JK, Kopecky KJ, Appelbaum FR, Head DR, Kingsbury LL, Balcerzak SP, et al. (October 1996). "A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study". Blood. 88 (8): 2841–51. doi:10.1182/blood.V88.8.2841.bloodjournal8882841. PMID 8874180.
- Bishop JF, Matthews JP, Young GA, Szer J, Gillett A, Joshua D, et al. (March 1996). "A randomized study of high-dose cytarabine in induction in acute myeloid leukemia". Blood. 87 (5): 1710–7. doi:10.1182/blood.V87.5.1710.1710. PMID 8634416.
- Iland HJ, Seymour JF (June 2013). "Role of arsenic trioxide in acute promyelocytic leukemia". Current Treatment Options in Oncology. 14 (2): 170–84. doi:10.1007/s11864-012-0223-3. hdl:11343/219801. PMID 23322117.
- Alimoghaddam K (July 2014). "A review of arsenic trioxide and acute promyelocytic leukemia". International Journal of Hematology-Oncology and Stem Cell Research. 8 (3): 44–54. PMC 4305381. PMID 25642308.
- Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, et al. (August 1988). "Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia". Blood. 72 (2): 567–72. doi:10.1182/blood.V72.2.567.567. PMID 3165295.
- Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, et al. (October 1997). "All-trans-retinoic acid in acute promyelocytic leukemia". The New England Journal of Medicine. 337 (15): 1021–8. doi:10.1056/NEJM199710093371501. PMID 9321529.
- Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E, et al. (August 1999). "A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group". Blood. 94 (4): 1192–200. doi:10.1182/blood.V94.4.1192. PMID 10438706.
- Estey EH (March 2002). "Treatment of acute myelogenous leukemia". Oncology. 16 (3): 343–52, 355–6, discussion 357, 362, 365–6. PMID 15046392.
- Cassileth PA, Harrington DP, Hines JD, Oken MM, Mazza JJ, McGlave P, et al. (April 1988). "Maintenance chemotherapy prolongs remission duration in adult acute nonlymphocytic leukemia". Journal of Clinical Oncology. 6 (4): 583–7. doi:10.1200/JCO.1988.6.4.583. PMID 3282032.
- Küley-Bagheri Y, Kreuzer KA, Monsef I, Lübbert M, Skoetz N, et al. (Cochrane Haematological Malignancies Group) (August 2018). "Effects of all-trans retinoic acid (ATRA) in addition to chemotherapy for adults with acute myeloid leukaemia (AML) (non-acute promyelocytic leukaemia (non-APL))". The Cochrane Database of Systematic Reviews. 8: CD011960. doi:10.1002/14651858.CD011960.pub2. PMID 30080246.
- Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. (October 1994). "Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B". The New England Journal of Medicine. 331 (14): 896–903. doi:10.1056/NEJM199410063311402. PMID 8078551.
- Appelbaum FR, Baer MR, Carabasi MH, Coutre SE, Erba HP, Estey E, et al. (November 2000). "NCCN Practice Guidelines for Acute Myelogenous Leukemia". Oncology. 14 (11A): 53–61. PMID 11195419.
- Brune M, Castaigne S, Catalano J, Gehlsen K, Ho AD, Hofmann WK, et al. (July 2006). "Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial". Blood. 108 (1): 88–96. doi:10.1182/blood-2005-10-4073. PMID 16556892.
- Abeloff, Martin et al. (2004), pp. 2840–41.
- Appelbaum FR (April 2001). "Who should be transplanted for AML?". Leukemia. 15 (4): 680–2. doi:10.1038/sj/leu/2402074. PMID 11368380.
- Appelbaum FR (February 2002). "Hematopoietic cell transplantation beyond first remission". Leukemia. 16 (2): 157–9. doi:10.1038/sj.leu.2402345. PMID 11840278.
- Sievers EL, Larson RA, Stadtmauer EA, Estey E, Löwenberg B, Dombret H, et al. (July 2001). "Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse". Journal of Clinical Oncology. 19 (13): 3244–54. doi:10.1200/JCO.2001.19.13.3244. PMID 11432892.
- http://www.pfizer.com/files/products/mylotarg_hcp_letter.pdf
- "Press Announcements - FDA approves Mylotarg for treatment of acute myeloid leukemia". 30 November 2018.
- Knips L, Bergenthal N, Streckmann F, Monsef I, Elter T, Skoetz N, et al. (Cochrane Haematological Malignancies Group) (January 2019). "Aerobic physical exercise for adult patients with haematological malignancies". The Cochrane Database of Systematic Reviews. 1: CD009075. doi:10.1002/14651858.CD009075.pub3. PMID 30702150.
- Fisher SA, Cutler A, Doree C, Brunskill SJ, Stanworth SJ, Navarrete C, Girdlestone J, et al. (Cochrane Haematological Malignancies Group) (January 2019). "Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition". The Cochrane Database of Systematic Reviews. 1: CD009768. doi:10.1002/14651858.CD009768.pub2. PMID 30697701.
- Estcourt L, Stanworth S, Doree C, Hopewell S, Murphy MF, Tinmouth A, Heddle N, et al. (Cochrane Haematological Malignancies Group) (May 2012). "Prophylactic platelet transfusion for prevention of bleeding in patients with haematological disorders after chemotherapy and stem cell transplantation". The Cochrane Database of Systematic Reviews (5): CD004269. doi:10.1002/14651858.CD004269.pub3. PMID 22592695.
- Estcourt LJ, Stanworth SJ, Doree C, Hopewell S, Trivella M, Murphy MF, et al. (Cochrane Haematological Malignancies Group) (November 2015). "Comparison of different platelet count thresholds to guide administration of prophylactic platelet transfusion for preventing bleeding in people with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation". The Cochrane Database of Systematic Reviews (11): CD010983. doi:10.1002/14651858.CD010983.pub2. PMID 26576687.
- Estey EH (April 2001). "Prognostic factors in acute myelogenous leukemia". Leukemia. 15 (4): 670–2. doi:10.1038/sj/leu/2402057. PMID 11368376.
- Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, et al. (October 1999). "A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties". British Journal of Haematology. 107 (1): 69–79. doi:10.1046/j.1365-2141.1999.01684.x. PMID 10520026.
- Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. (December 2000). "Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study". Blood. 96 (13): 4075–83. doi:10.1182/blood.V96.13.4075. PMID 11110676.
- Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. (December 2002). "Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461)". Blood. 100 (13): 4325–36. doi:10.1182/blood-2002-03-0772. PMID 12393746. S2CID 16003833.
- Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. (October 1998). "The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties". Blood. 92 (7): 2322–33. doi:10.1182/blood.V92.7.2322. PMID 9746770.
- Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. (December 2000). "Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study". Blood. 96 (13): 4075–83. doi:10.1182/blood.V96.13.4075. PMID 11110676.
- Thirman MJ, Larson RA (April 1996). "Therapy-related myeloid leukemia". Hematology/Oncology Clinics of North America. 10 (2): 293–320. doi:10.1016/S0889-8588(05)70340-3. PMID 8707757.
- Rowley JD, Golomb HM, Vardiman JW (October 1981). "Nonrandom chromosome abnormalities in acute leukemia and dysmyelopoietic syndromes in patients with previously treated malignant disease". Blood. 58 (4): 759–67. doi:10.1182/blood.V58.4.759.759. PMID 7272506.
- Pedersen-Bjergaard J, Andersen MK, Christiansen DH, Nerlov C (March 2002). "Genetic pathways in therapy-related myelodysplasia and acute myeloid leukemia". Blood. 99 (6): 1909–12. doi:10.1182/blood.V99.6.1909. PMID 11877259. S2CID 15397577.
- Haferlach T, Schoch C, Löffler H, Gassmann W, Kern W, Schnittger S, et al. (January 2003). "Morphologic dysplasia in de novo acute myeloid leukemia (AML) is related to unfavorable cytogenetics but has no independent prognostic relevance under the conditions of intensive induction therapy: results of a multiparameter analysis from the German AML Cooperative Group studies". Journal of Clinical Oncology. 21 (2): 256–65. doi:10.1200/JCO.2003.08.005. PMID 12525517.
- Chabner BA, Lynch TJ, Longo DL (22 March 2014). Harrisons Manual of Oncology 2/E. McGraw Hill Professional. p. 294. ISBN 9780071793261.
- Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta E, et al. (December 1998). "Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission". The New England Journal of Medicine. 339 (23): 1649–56. doi:10.1056/NEJM199812033392301. PMID 9834301.
- Matthews JP, Bishop JF, Young GA, Juneja SK, Lowenthal RM, Garson OM, et al. (June 2001). "Patterns of failure with increasing intensification of induction chemotherapy for acute myeloid leukaemia". British Journal of Haematology. 113 (3): 727–36. doi:10.1046/j.1365-2141.2001.02756.x. PMID 11380464.
- Sanz MA, Lo Coco F, Martín G, Avvisati G, Rayón C, Barbui T, et al. (August 2000). "Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups". Blood. 96 (4): 1247–53. PMID 10942364. Archived from the original on 27 May 2010. Retrieved 17 March 2008.
- Duffin J (5 September 2016). "Pondering Miracles, Medical and Religious". The New York Times. ISSN 0362-4331. Retrieved 12 March 2018.
- Jemal A, Thomas A, Murray T, Thun M (2002). "Cancer statistics, 2002". Ca. 52 (1): 23–47. doi:10.3322/canjclin.52.1.23. PMID 11814064.
- Leone G, Mele L, Pulsoni A, Equitani F, Pagano L (October 1999). "The incidence of secondary leukemias". Haematologica. 84 (10): 937–45. PMID 10509043.
- Greenlee RT, Hill-Harmon MB, Murray T, Thun M (2001). "Cancer statistics, 2001". Ca. 51 (1): 15–36. doi:10.3322/canjclin.51.1.15. PMID 11577478.
- Linet MS (1985). "The leukemias: Epidemiologic aspects.". In Lilienfeld AM (ed.). Monographs in Epidemiology and Biostatistics. New York: Oxford University Press. p. I. ISBN 978-0-19-503448-6.
- Aoki K, Kurihars M, Hayakawa N (1992). Death Rates for Malignant Neoplasms for Selected Sites by Sex and Five-Year Age Group in 33 Countries 1953–57 to 1983–87. Nagoya, Japan: University of Nagoya Press, International Union Against Cancer.
- Bhatia S, Neglia JP (May 1995). "Epidemiology of childhood acute myelogenous leukemia". Journal of Pediatric Hematology/Oncology. 17 (2): 94–100. doi:10.1097/00043426-199505000-00002. PMID 7749772.
- "Acute myeloid leukaemia (AML) statistics". Cancer Research UK. Retrieved 27 October 2014.
- Bennett JH (1845). "Two cases of hypertrophy of the spleen and liver, in which death took place from suppuration of blood". Edinburgh Med Surg J. 64: 413.
- Virchow R (1856). "Die Leukämie". In Virchow R (ed.). Gesammelte Abhandlungen zur Wissenschaftlichen Medizin (in German). Frankfurt: Meidinger. p. 190.
- Ebstein W (1889). "Über die acute Leukämie und Pseudoleukämie". Deutsch Arch Klin Med. 44: 343.
- Mosler F (1876). "Klinische Symptome und Therapie der medullären Leukämie". Berl Klin Wochenschr. 13: 702.
- Naegeli O (1900). "Über rothes Knochenmark und Myeloblasten". Deutsche Medizinische Wochenschrift. 26 (18): 287–290. doi:10.1055/s-0029-1203820.
- Wang ZY (2003). "Ham-Wasserman lecture: treatment of acute leukemia by inducing differentiation and apoptosis". Hematology. American Society of Hematology. Education Program. 2003 (1): 1–13. doi:10.1182/asheducation-2003.1.1. PMID 14633774.
- Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K, et al. (November 2008). "DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome". Nature. 456 (7218): 66–72. Bibcode:2008Natur.456...66L. doi:10.1038/nature07485. PMC 2603574. PMID 18987736.
- Shapira T, Pereg D, Lishner M (September 2008). "How I treat acute and chronic leukemia in pregnancy". Blood Reviews. 22 (5): 247–59. doi:10.1016/j.blre.2008.03.006. PMID 18472198.
External links
| Classification | |
|---|---|
| External resources |