Threonine
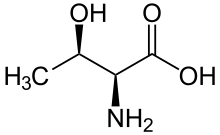
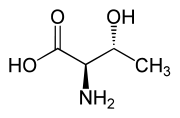
Threonine (symbol Thr or T)[2] is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as E. coli.[3] It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG).
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Threonine | |
| Other names
2-Amino-3-hydroxybutanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.704 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H9NO3 | |
| Molar mass | 119.120 g·mol−1 |
| (H2O, g/dl) 10.6(30°),14.1(52°),19.0(61°) | |
| Acidity (pKa) | 2.63 (carboxyl), 10.43 (amino)[1] |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices).
Modifications
The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can undergo O-linked glycosylation. In addition, threonine residues undergo phosphorylation through the action of a threonine kinase. In its phosphorylated form, it can be referred to as phosphothreonine. Phosphothreonine has three potential coordination sites (carboxyl, amine and phosphate group) and determination of the mode of coordination between phosphorylated ligands and metal ions occurring in an organism is important to explain the function of the phosphothreonine in biological processes.[4]
It is a precursor of glycine, and can be used as a prodrug to reliably elevate brain glycine levels.
History
Threonine was the last of the 20 common proteinogenic amino acids to be discovered. It was discovered in 1936 by William Cumming Rose[5], collaborating with Curtis Meyer. The amino acid was named threonine because it was similar in structure to threonic acid, a four-carbon monosaccharide with molecular formula C4H8O5[6]
 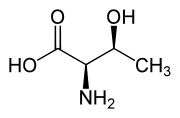 |
| L-Threonine (2S,3R) and D-Threonine (2R,3S) |
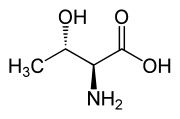  |
| L-Allothreonine (2S,3S) and D-Allothreonine (2R,3R) |
Threonine is one of two proteinogenic amino acids with two chiral centers, the other being isoleucine. Threonine can exist in four possible stereoisomers with the following configurations: (2S,3R), (2R,3S), (2S,3S) and (2R,3R). However, the name L-threonine is used for one single diastereomer, (2S,3R)-2-amino-3-hydroxybutanoic acid. The second stereoisomer (2S,3S), which is rarely present in nature, is called L-allothreonine.[7] The two stereoisomers (2R,3S)- and (2R,3R)-2-amino-3-hydroxybutanoic acid are only of minor importance.
Biosynthesis
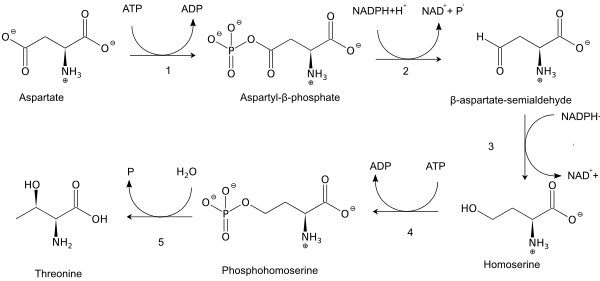
As an essential amino acid, threonine is not synthesized in humans, and needs to be present in proteins in the diet. Adult humans require about 20 mg/kg body weight/day.[8] In plants and microorganisms, threonine is synthesized from aspartic acid via α-aspartyl-semialdehyde and homoserine. Homoserine undergoes O-phosphorylation; this phosphate ester undergoes hydrolysis concomitant with relocation of the OH group.[9] Enzymes involved in a typical biosynthesis of threonine include:
- aspartokinase
- β-aspartate semialdehyde dehydrogenase
- homoserine dehydrogenase
- homoserine kinase
- threonine synthase.
Metabolism
Threonine is metabolized in at least three ways:
- In many animals it is converted to pyruvate via threonine dehydrogenase. An intermediate in this pathway can undergo thiolysis with CoA to produce acetyl-CoA and glycine.
- In humans the gene for threonine dehydrogenase is an inactive pseudogene[10], so threonine is converted to α-ketobutyrate. The mechanism of the first step is analogous to that catalyzed by serine dehydratase, and the serine and threonine dehydratase reactions are probably catalyzed by the same enzyme.[11]
- In many organisms it is O-phosphorylated by a kinase preparatory to further metabolism. This is especially important in bacteria as part of the biosynthesis of cobalamin (Vitamin B12), as the product is converted to (R)-1-aminopropan-2-ol for incorporation into the vitamin's sidechain.[12]
Sources
Foods high in threonine include cottage cheese, poultry, fish, meat, lentils, black turtle bean[13] and sesame seeds.[14]
Racemic threonine can be prepared from crotonic acid by alpha-functionalization using mercury(II) acetate.[15]
References
- Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
- Raïs, Badr; Chassagnole, Christophe; Lettelier, Thierry; Fell, David; Mazat, Jean-Pierre (2001). "Threonine synthesis from aspartate in Escherichia coli cell-free extracts: pathway dynamics". J Biochem. 356 (Pt 2): 425–32. doi:10.1042/bj3560425. PMC 1221853. PMID 11368769.
- Jastrzab, Renata (2013). "Studies of new phosphothreonine complexes formed in binary and ternary systems including biogenic amines and copper(II)". Journal of Coordination Chemistry. 66 (1): 98-113. doi:10.1080/00958972.2012.746678
- A Dictionary of scientists. Daintith, John., Gjertsen, Derek. Oxford: Oxford University Press. 1999. p. 459. ISBN 9780192800862. OCLC 44963215.CS1 maint: others (link)
- Meyer, Curtis (20 July 1936). "The Spatial Configuation of Alpha-Amino-Beta-Hydroxy-n-Butyric Acid" (PDF). Journal of Biological Chemistry. 115 (3).
- "Nomenclature and symbolism for amino acids and peptides (Recommendations 1983)". Pure and Applied Chemistry. 56 (5): 601, 603, 608. 1 January 1984. doi:10.1351/pac198456050595.
- Institute of Medicine (2002). "Protein and Amino Acids". Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. pp. 589–768.
- Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2000). Principles of Biochemistry (3rd ed.). New York: W. H. Freeman. ISBN 1-57259-153-6..
- Stipanuk, Martha H.; Caudill, Marie A. (2013-08-13). Biochemical, Physiological, and Molecular Aspects of Human Nutrition - E-Book. Elsevier Health Sciences. ISBN 9780323266956.
- Bhardwaj, Uma; Bhardwaj, Ravindra. Biochemistry for Nurses. Pearson Education India. ISBN 9788131795286.
- Fang, H; Kang, J; Zhang, D (30 January 2017). "Microbial production of vitamin B12: a review and future perspectives". Microbial Cell Factories. 16 (1): 15. doi:10.1186/s12934-017-0631-y. PMC 5282855. PMID 28137297.
- "Error". ndb.nal.usda.gov.
- "SELF Nutrition Data - Food Facts, Information & Calorie Calculator". nutritiondata.self.com. Retrieved 27 March 2018.
- Carter, Herbert E.; West, Harold D. (1940). "dl-Threonine". Organic Syntheses. 20: 101.; Collective Volume, 3, p. 813.
