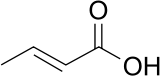
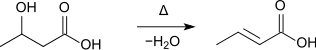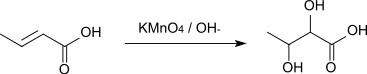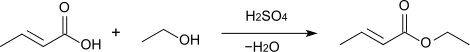Crotonic acid
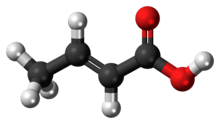
Crotonic acid ((2E)-but-2-enoic acid) is a short-chain unsaturated carboxylic acid, described by the formula CH3CH=CHCO2H. It is called crotonic acid because it was erroneously thought to be a saponification product of croton oil.[2] It crystallizes as colorless needles from hot water. The cis-isomer of crotonic acid is called isocrotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to butyric acid.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-But-2-enoic acid | |
| Other names
(E)-But-2-enoic acid (E)-2-Butenoic acid Crotonic acid trans-2-Butenoic acid β-Methylacrylic acid 3-Methylacrylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.213 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6O2 | |
| Molar mass | 86.090 g·mol−1 |
| Density | 1.02 g/cm3 |
| Melting point | 70 to 73 °C (158 to 163 °F; 343 to 346 K) |
| Boiling point | 185 to 189 °C (365 to 372 °F; 458 to 462 K) |
| Acidity (pKa) | 4.69 [1] |
| Hazards | |
| Safety data sheet | SIRI.org |
| Related compounds | |
Other anions |
crotonate |
Related carboxylic acids |
propionic acid acrylic acid butyric acid succinic acid malic acid tartaric acid fumaric acid pentanoic acid tetrolic acid |
Related compounds |
butanol butyraldehyde crotonaldehyde 2-butanone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
Crotonic acid may be obtained by several methods:
- by oxidation of crotonaldehyde:[3]:230
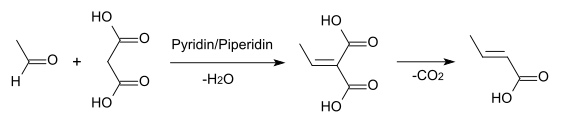
- by Knoevenagel condensation of acetaldehyde with malonic acid in pyridine:[3]:229
- or by alkaline hydrolysis of allyl cyanide after the intramolecular rearrangement of the double bond:[4][5]
- Furthermore, it is formed during the distillation of 3-hydroxybutyric acid:[6]
Properties
Crotonic acid crystallizes in the monoclinic crystal system in the space group P21/a (space group 14, position 3) with the lattice parameters a = 971 pm, b = 690 pm, c = 775 pm and β = 104.0°. The unit cell contains four formula units.[7]
Reactions
Crotonic acid converts into butyric acid by hydrogenation or by reduction with zinc and sulfuric acid.[8]
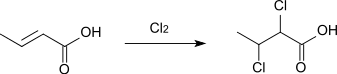
Upon treatment with chlorine or bromine, crotonic acid converts to 2,3-dihalobutyric acids:[8]
Crotonic acid adds hydrogen bromide to form 3-bromobutyric acid.[8][9]
The reaction with alkaline potassium permanganate solution affords 2,3-dihydroxybutyric acid.[8]
Upon heating with acetic anhydride, crotonic acid converts to the acid anhydride:[10]
Esterification of crotonic acid using sulfuric acid as a catalyst provides the corresponding crotonate esters:
Crotonic acid reacts with hypochlorous acid to 2-chloro-3-hydroxybutyric acid. This can either be reduced with sodium amalgam to butyric acid, can form with sulfuric acid 2-chlorobutenoic acid, react with hydrogen chloride to 2,3-dichlorobutenoic acid or with potassium ethoxide to 3-methyloxirane-2-carboxylic acid.[11]
Crotonic acid reacts with ammonia at the alpha position in the presence of mercury(II) acetate. This reaction provides DL-threonine.[12]
Use
Crotonic acid is mainly used as a comonomer with vinyl acetate.[13] The resulting copolymers are used in paints and adhesives.[14]
Crotonyl chloride reacts with N-ethyl-2-methylaniline (N-ethyl-o-toluidine) to provide crotamiton, which is used as an agent against scabies.[15]
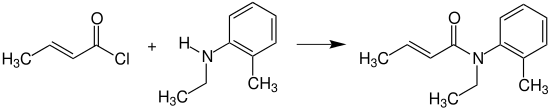 Crotamiton synthesis
Crotamiton synthesis
Safety
Its LD50 is 1 g/kg (oral, rats).[14] It irritates eyes, skin, and respiratory system.[13]
References
- Dawson, R. M. C.; et al. (1959). Data for Biochemical Research. Oxford: Clarendon Press.
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 7 (11th ed.). Cambridge University Press. p. 511.
- Beyer, Hans; Walter, Wolfgang (1984). Organische Chemie (in German). Stuttgart: S. Hirzel Verlag. ISBN 3-7776-0406-2.
- Rinne, A.; Tollens, B. (1871). "Ueber das Allylcyanür oder Crotonitril" [On allyl cyanide or crotononitrile]. Justus Liebigs Annalen der Chemie. 159 (1): 105–109. doi:10.1002/jlac.18711590110.
- Pomeranz, C. (1906). "Ueber Allylcyanid und Allylsenföl" [On allyl cyanide and allylic mustard oil]. Justus Liebigs Annalen der Chemie. 351 (1–3): 354–362. doi:10.1002/jlac.19073510127.
- Beilstein, F. (1893). Handbuch der organischen Chemie (in German). 1 (3rd ed.). Verlag Leopold Voss. p. 506.
- Shimizu, S.; Kekka, S.; Kashino, S.; Haisa, M. (1974). "Topochemical Studies. III. The Crystal and Molecular Structures of Crotonic Acid, CH3CH=CHCO2H, and Crotonamide, CH3CH=CHCONH2". Bulletin of the Chemical Society of Japan. 47 (7): 1627–1631. doi:10.1246/bcsj.47.1627.
- Heilbron (1953). "Crotonic acid". Dictionary of Organic Compounds. 1: 615.
- Lovén, J. M.; Johansson, H. (1915). "Einige schwefelhaltige β-Substitutionsderivate der Buttersäure" [Some sulfur-containing β-substitution derivatives of butyric acid]. Berichte der deutschen chemischen Gesellschaft. 48 (2): 1254–1262. doi:10.1002/cber.19150480205.
- Clover, A. M.; Richmond, G. F. (1903). "The Hydrolysis of Organic Peroxides and Peracids". American Chemical Journal. 29 (3): 179–203.
- Beilstein, F. (1893). Handbuch der organischen Chemie (in German). 1 (3rd ed.). Verlag Leopold Voss. p. 562.
- Carter, H. E.; West, H. D. (1955). "dl-Threonine". Organic Syntheses.; Collective Volume, 3, p. 813
- Entry on Butensäuren. at: Römpp Online. Georg Thieme Verlag, retrieved January 7, 2020.
- Schulz, R. P.; Blumenstein, J.; Kohlpaintner, C. (2005). "Crotonaldehyde and Crotonic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_083.
- Kleemann, A.; Engel, J. Pharmazeutische Wirkstoffe: Synthesen, Patente, Anwendungen. 5 (2nd rev. and updated ed.). Stuttgart & New York: Georg Thieme Verlag. p. 251. ISBN 3-13-558402-X.