Gallium(III) oxide
Gallium(III) trioxide is an inorganic compound with the formula Ga2O3. It exists as several polymorphs, all of which are white, water-insoluble solids. Although no commercial applications exist, Ga2O3 is an intermediate in the purification of gallium, which is consumed almost exclusively as gallium arsenide.[6]
_oxide_crystal.jpg) β-Ga2O3 crystal | |
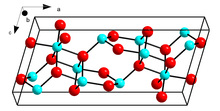 Crystal structure of β-Ga2O3 | |
| Names | |
|---|---|
| Other names
gallium trioxide, gallium sesquioxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.525 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Ga2O3 | |
| Molar mass | 187.444 g/mol[1] |
| Appearance | white crystalline powder |
| Density | 6.44 g/cm3, alpha 5.88 g/cm3, beta |
| Melting point | 1,900 °C (3,450 °F; 2,170 K) alpha 1725 °C, beta [2] |
| insoluble | |
| Solubility | soluble in most acids |
| Structure | |
| α: Trigonal, hR30, space group = R3c, No. 167[3] β: Monoclinic, mS20, space group = C2/m, No. 12[4] | |
a = 0.49835 / 1.22247 nm, b = 0.49835 / 0.30403 nm, c = 0.53286 / 0.58088 nm | |
Formula units (Z) |
6 / 4 |
| Thermochemistry | |
Heat capacity (C) |
92.1 J/mol·K[5] |
Std molar entropy (S |
85.0 J/mol·K[5] |
Std enthalpy of formation (ΔfH⦵298) |
−1089.1 kJ/mol[5] |
Gibbs free energy (ΔfG˚) |
-998.3 kJ/mol[5] |
| Hazards | |
EU classification (DSD) (outdated) |
not listed |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Gallium trioxide is precipitated in hydrated form upon neutralization of acidic or basic solution of gallium salt. Also, it is formed on heating gallium in air or by thermally decomposing gallium nitrate at 200–250 ˚C. It can occur in five different modifications, α, β, γ, δ, and ε. Of these modifications β-Ga2O3 is the most stable form.[7]
- β-Ga2O3 is prepared by heating nitrate, acetate, oxalate or other organic derivatives above 1000 °C.
- α-Ga2O3 can be obtained by heating β-Ga2O3 at 65 kbars and 1100 °C. The hydrated form can be prepared by decomposing precipitated and "aged" gallium hydroxide at 500 °C.
- γ-Ga2O3 is prepared by rapidly heating the hydroxide gel at 400–500 °C. A more crystalline form of this polymorph can be prepared directly from gallium metal by a solvothermal synthesis.[8]
- δ-Ga2O3 is obtained by heating Ga(NO3)3 at 250 °C.
- ε-Ga2O3 is prepared by heating δ-Ga2O3 at 550 °C.[7] Thin films of ε-Ga2O3 are deposited by means of Metalorganic vapour phase epitaxy using Trimethylgallium and water on sapphire substrates at temperatures between 550 and 650 °C [9]
Reactions
Gallium(III) trioxide is amphoteric.[10] It reacts with alkali metal oxides at high temperature to form, e.g., NaGaO2, and with Mg, Zn, Co, Ni, Cu oxides to form spinels, e.g. MgGa2O4.[11]
It dissolves in strong alkali to form a solution of the gallate ion, Ga(OH)−
4.
With HCl, it forms gallium trichloride GaCl3.[12]
- Ga2O3 + 6 HCl → 2 GaCl3 + 3 H2O
It can be reduced to gallium suboxide (gallium(I) oxide) Ga2O by H2.[13] or by reaction with gallium metal:[14]
- Ga2O3 + 2 H2 → Ga2O + 2 H2O
- Ga2O3 + 4 Ga → 3 Ga2O
Structure
β-Ga2O3, with a melting point of 1900 ˚C, is the most stable crystalline modification. The oxide ions are in a distorted cubic closest packing arrangement, and the gallium (III) ions occupy distorted tetrahedral and octahedral sites, with Ga-O bond distances of 1.83 and 2.00 Å respectively.[15]
α-Ga2O3 has the same structure (corundum) as α-Al2O3, wherein Ga ions are 6-coordinate. γ-Ga2O3 has a defect spinel structure similar to that of γ-Al2O3.[16]
ε-Ga2O3 films deposited by metalorganic vapour phase epitaxy show a columnar structure with orthorhombic crystal symmetry. Macroscopically, this structure is seen by X-ray crystallography as hexagonal close packed.[17]
Potential applications
Gallium(III) oxide has been studied in the use of lasers, phosphors, and luminescent materials.[7] It has also been used as an insulating barrier in tight junctions.[18] Monoclinic β-Ga2O3 is used in gas sensors and luminescent phosphors and can be applied to dielectric coatings for solar cells. This stable oxide has also shown potential for deep-ultraviolet transparent conductive oxides,[19] and transistor applications.[20][21]
ε-Ga2O3 thin films deposited on sapphire show potential applications as solar-blind UV photodetector.[22]
Thin Ga2O3 films are of commercial interest as gas sensitive materials and Ga2O3. Ellipsometry is a procedure that can be used to determine optical functions of the β-Ga2O3.[19]
β-Ga2O3 is used in the production of Ga2O3-Al2O3 catalyst.[23]
References
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.64. ISBN 1439855110.
- Patnaik, Pradyot (2002) Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 0-07-049439-8
- Eckert, L. J.; Bradt, R. C. (1973). "Thermal Expansion of Alpha Ga2O3". Journal of the American Ceramic Society. 56 (4): 229. doi:10.1111/j.1151-2916.1973.tb12471.x.
- Dohy, D.; Gavarri, J.R. (1983). "Oxyde β-Ga2O3: Champ de force, dilatation thermique, et rigidité anisotropes". Journal of Solid State Chemistry. 49 (1): 107–117. Bibcode:1983JSSCh..49..107D. doi:10.1016/0022-4596(83)90222-0.
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 5.12. ISBN 1439855110.
- Greber, J. F. (2012) "Gallium and Gallium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, doi:10.1002/14356007.a12_163.
- Bailar, J; Emeléus, H; Nyholm, R; Trotman-Dickenson, A. F. (1973). Comprehensive Inorganic Chemistry. Vol. 1, p. 1091
- Playford, Helen Y.; Hannon, Alex C.; Barney, Emma R.; Walton, Richard I. (2013). "Structures of Uncharacterised Polymorphs of Gallium triOxide from Total Neutron Diffraction". Chemistry – A European Journal. 19 (8): 2803–13. doi:10.1002/chem.201203359. PMID 23307528.
- Boschi, F.; Bosi, M.; Berzina, T.; Buffagni, E.; Ferrari, C.; Fornari, R. (2015). "Hetero-epitaxy of ε-Ga2O3 layers by MOCVD and ALD". Journal of Crystal Growth. 44: 25–30. doi:10.1016/j.jcrysgro.2016.03.013.
- Ebbing, Darrell D.; Gammon, Steven D. (2010) General Chemistry, 9th ed., Thomson Brooks/Cole. ISBN 0538497521
- Downs, Anthony John (ed.) (1993) The Chemistry of Aluminium, Gallium, Indium and Thallium. Springer . ISBN 075140103X
- Zuckerman, J J and Hagen, A P eds. (2009) Inorganic Reactions and Methods, the Formation of Bonds to Halogens (Part 2), Wiley-VCH Verlag GmbH, ISBN 9780470145395
- Koch, H. F.; Girard, L. A.; Roundhill, D. M. (1999). "Determination of Gallium in a Cerium Surrogate and in Drops from a Copper Collector by ICP as Model Studies for the Removal of Gallium from Plutonium". Atomic Spectroscopy. 20 (1): 30.
- Greenwood, N.N.; Emeleus, H. J. and Sharpe, A. G. (1963) "The chemistry of Gallium" in Advances in Inorganic Chemistry and Radiochemistry, Vol. 5, Elsevier, Academic Press
- King, R. B. (1994) Encyclopedia of Inorganic Chemistry. Vol. 3. p. 1256. ISBN 978-0-470-86078-6.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 247. ISBN 978-0-08-037941-8.
- Cora, I (2017). "The real structure of ε-Ga2O3 and its relation to κ-phase". CrystEngComm. 19 (11): 1509–1516. doi:10.1039/C7CE00123A.
- Dai, Z. R.; Pan, Z. W.; Wang, Z. L. (2002). "Gallium Oxide Nanoribbons and Nanosheets". The Journal of Physical Chemistry B. 106 (5): 902. CiteSeerX 10.1.1.655.6068. doi:10.1021/jp013228x.
- Rebien, M; Henrion, W; Hong, M; Mannaerts, J; Fleischer, M (2002). "Optical properties of gallium oxide thin films". Applied Physics Letters. 81 (2): 250. Bibcode:2002ApPhL..81..250R. doi:10.1063/1.1491613.
- Thomas, Stuart R.; Adamopoulos, George; Lin, Yen-Hung; Faber, Hendrik; Sygellou, Labrini; Stratakis, Emmanuel; Pliatsikas, Nikos; Patsalas, Panos A.; Anthopoulos, Thomas D (2014). "High electron mobility thin-film transistors based on Ga2O3 grown by atmospheric ultrasonic spray pyrolysis at low temperatures". Applied Physics Letters. 105 (9): 092105. Bibcode:2014ApPhL.105i2105T. doi:10.1063/1.4894643.
- Higashiwaki, M.; Jessen, G. H. (2018). "The dawn of gallium oxide microelectronics". Applied Physics Letters. 112 (6): 060401. doi:10.1063/1.5017845.
- Pavesi, M. (2018). "ε-Ga2O3 epilayers as a material for solar-blind UV photodetectors". Materials Chemistry and Physics. 205: 502–507. doi:10.1016/j.matchemphys.2017.11.023.
- Shimizu, Ken-Ichi; Takamatsu, Mikio; Nishi, Koji; Yoshida, Hisao; Satsuma, Atsushi; Tanaka, Tsunehiro; Yoshida, Satohiro; Hattori, Tadashi (1999). "Alumina-Supported Gallium trixide Catalysts for NO Selective Reduction: Influence of the Local Structure of Surface Gallium trioxide Species on the Catalytic Activity". The Journal of Physical Chemistry B. 103 (9): 1542. doi:10.1021/jp983790w.