Epidermal growth factor
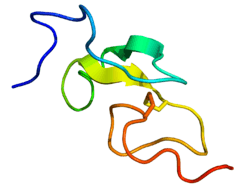
Epidermal growth factor (EGF) is a protein that stimulates cell growth and differentiation by binding to its receptor, EGFR. Human EGF is 6-kDa[5] and has 53 amino acid residues and three intramolecular disulfide bonds.[6]
EGF was originally described as a secreted peptide found in the submaxillary glands of mice and in human urine. EGF has since been found in many human tissues including submandibular gland (submaxillary gland) and parotid gland.[7] Initially, human EGF was known as urogastrone.[8]
Function
EGF, via binding to its cognate receptor, results in cellular proliferation, differentiation, and survival.[9]
Salivary EGF, which seems to be regulated by dietary inorganic iodine, also plays an important physiological role in the maintenance of oro-esophageal and gastric tissue integrity. The biological effects of salivary EGF include healing of oral and gastroesophageal ulcers, inhibition of gastric acid secretion, stimulation of DNA synthesis as well as mucosal protection from intraluminal injurious factors such as gastric acid, bile acids, pepsin, and trypsin and to physical, chemical and bacterial agents.[7]
Biological sources
Epidermal growth factor can be found in urine, saliva, milk, tears, and plasma.[10] The production of epidermal growth factor has been found to be stimulated by testosterone.
Selected Polypeptide Growth Factors[11]
| Sr.No | Growth factor | Source | Major function |
|---|---|---|---|
| 1 | Epidermal growth factor | Salivary gland | Stimulate growth of epidermal and epithelial cell |
| 2 | Platelet derived growth factor | Platelets | Stimulate growth of mesenchymal cells, promotes wound healing |
| 3 | Transforming growth factor (Alpha) | Epithelial cell | Similar to Epidermal growth factor |
| 4 | Transforming growth factor (Beta) | Platelets, Kidney, Placenta | Inhibitory effect on cultures tumor cell |
| 5 | Erythropoietin | Kidney | Stimulate development of erythropoietic cells |
| 6 | Nerve growth factor (NGF) | Salivary gland | Stimulate the growth of sensory nerve |
| 7 | Insulin like growth factor | Serum | Stimulate incorporation of sulfates into cartilage, exerts insulin like action on certain cell |
| 8 | Tumor necrosis factor | Monocytes | Necrosis of tumor cells |
| 9 | Interleukin-1 | Monocytes, Leukocytes | Stimulate synthesis of IL-2 |
| 10 | Interleukin-2 | Lymphocytes | Stimulate growth and maturation of T-cells |
Mechanism
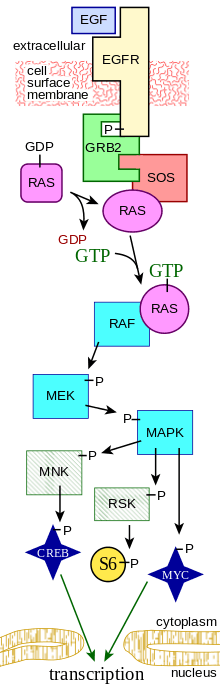
EGF acts by binding with high affinity to epidermal growth factor receptor (EGFR) on the cell surface. This stimulates ligand-induced dimerization,[12] activating the intrinsic protein-tyrosine kinase activity of the receptor (see the second diagram). The tyrosine kinase activity, in turn, initiates a signal transduction cascade that results in a variety of biochemical changes within the cell – a rise in intracellular calcium levels, increased glycolysis and protein synthesis, and increases in the expression of certain genes including the gene for EGFR – that ultimately lead to DNA synthesis and cell proliferation.[13]
EGF-family / EGF-like domain
EGF is the founding member of the EGF-family of proteins. Members of this protein family have highly similar structural and functional characteristics. Besides EGF itself other family members include:[14]
- Heparin-binding EGF-like growth factor (HB-EGF)
- transforming growth factor-α (TGF-α)
- Amphiregulin (AR)
- Epiregulin (EPR)
- Epigen
- Betacellulin (BTC)
- neuregulin-1 (NRG1)
- neuregulin-2 (NRG2)
- neuregulin-3 (NRG3)
- neuregulin-4 (NRG4).
All family members contain one or more repeats of the conserved amino acid sequence:
CX7CX4-5CX10-13CXCX8GXRC
Where C is cysteine, G is glycine, R is arginine, and X represents any amino acid.[14]
This sequence contains six cysteine residues that form three intramolecular disulfide bonds. Disulfide bond formation generates three structural loops that are essential for high-affinity binding between members of the EGF-family and their cell-surface receptors.[5]
Interactions
Epidermal growth factor has been shown to interact with epidermal growth factor receptor.[15][16]
Medical uses
Recombinant human epidermal growth factor, sold under the brand name Heberprot-P, is used to treat diabetic foot ulcers. It can be given by injection into the wound site,[17] or may be used topically.[18] Tentative evidence shows improved wound healing.[19] Safety has been poorly studied.[19]
EGF is used to modify synthetic scaffolds for manufacturing of bioengineered grafts by emulsion electrospinning or surface modification methods.[20][21]
Bone regeneration
EGF plays an enhancer role on osteogenic differentiation of dental pulp stem cells (DPSCs) because it is capable of increasing extracellular matrix mineralization. A low concentration of EGF (10 ng/ml) is sufficient to induce morphological and phenotypic changes. These data suggests that DPSCs in combination with EGF could be an effective stem cell-based therapy to bone tissue engineering applications in periodontics and oral implantology.[22]
References
- GRCh38: Ensembl release 89: ENSG00000138798 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000028017 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Harris RC, Chung E, Coffey RJ (March 2003). "EGF receptor ligands". Experimental Cell Research. 284 (1): 2–13. doi:10.1016/S0014-4827(02)00105-2. PMID 12648462.
- Carpenter G, Cohen S (May 1990). "Epidermal growth factor". The Journal of Biological Chemistry. 265 (14): 7709–12. PMID 2186024.
- Venturi S, Venturi M (2009). "Iodine in evolution of salivary glands and in oral health". Nutrition and Health. 20 (2): 119–34. doi:10.1177/026010600902000204. PMID 19835108.
- Hollenberg MD, Gregory H (May 1980). "Epidermal growth factor-urogastrone: biological activity and receptor binding of derivatives". Molecular Pharmacology. 17 (3): 314–20. PMID 6248761.
- Herbst RS (2004). "Review of epidermal growth factor receptor biology". International Journal of Radiation Oncology, Biology, Physics. 59 (2 Suppl): 21–6. doi:10.1016/j.ijrobp.2003.11.041. PMID 15142631.
- Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. ISBN 978-0-7216-0187-8.
- Satyanarayana, U. (2002). Biochemistry (2nd ed.). Kolkata, India: Books and Allied. ISBN 8187134801. OCLC 71209231.
- Dawson JP, Berger MB, Lin CC, Schlessinger J, Lemmon MA, Ferguson KM (September 2005). "Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface". Molecular and Cellular Biology. 25 (17): 7734–42. doi:10.1128/MCB.25.17.7734-7742.2005. PMC 1190273. PMID 16107719.
- Fallon JH, Seroogy KB, Loughlin SE, Morrison RS, Bradshaw RA, Knaver DJ, Cunningham DD (June 1984). "Epidermal growth factor immunoreactive material in the central nervous system: location and development". Science. 224 (4653): 1107–9. doi:10.1126/science.6144184. PMID 6144184.
- Dreux AC, Lamb DJ, Modjtahedi H, Ferns GA (May 2006). "The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis". Atherosclerosis. 186 (1): 38–53. doi:10.1016/j.atherosclerosis.2005.06.038. PMID 16076471.
- Stortelers C, Souriau C, van Liempt E, van de Poll ML, van Zoelen EJ (July 2002). "Role of the N-terminus of epidermal growth factor in ErbB-2/ErbB-3 binding studied by phage display". Biochemistry. 41 (27): 8732–41. doi:10.1021/bi025878c. PMID 12093292.
- Wong L, Deb TB, Thompson SA, Wells A, Johnson GR (March 1999). "A differential requirement for the COOH-terminal region of the epidermal growth factor (EGF) receptor in amphiregulin and EGF mitogenic signaling". The Journal of Biological Chemistry. 274 (13): 8900–9. doi:10.1074/jbc.274.13.8900. PMID 10085134.
- Berlanga J, Fernández JI, López E, López PA, del Río A, Valenzuela C, Baldomero J, Muzio V, Raíces M, Silva R, Acevedo BE, Herrera L (January 2013). "Heberprot-P: a novel product for treating advanced diabetic foot ulcer". MEDICC Review. 15 (1): 11–5. doi:10.1590/s1555-79602013000100004. PMID 23396236.
- Yang S, Geng Z, Ma K, Sun X, Fu X (June 2016). "Efficacy of Topical Recombinant Human Epidermal Growth Factor for Treatment of Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis". The International Journal of Lower Extremity Wounds. 15 (2): 120–5. doi:10.1177/1534734616645444. PMID 27151755.
- Martí-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, Oliva P, Cedeño-Taborda J (October 2015). "Growth factors for treating diabetic foot ulcers". The Cochrane Database of Systematic Reviews. 10 (10): CD008548. doi:10.1002/14651858.CD008548.pub2. PMID 26509249.
- Haddad T, Noel S, Liberelle B, El Ayoubi R, Ajji A, De Crescenzo G (January 2016). "Fabrication and surface modification of poly lactic acid (PLA) scaffolds with epidermal growth factor for neural tissue engineering". Biomatter. 6 (1): e1231276. doi:10.1080/21592535.2016.1231276. PMC 5098722. PMID 27740881.
- Tenchurin, T.H.; Lyundup, A.V.; Demchenko, A.G.; Krasheninnikov, M.E.; Balyasin, M.V.; Klabukov, I.D.; Shepelev, A.D.; Mamagulashvili, V.G.; Orehov, A.S. (2017). "Modification of biodegradable fibrous scaffolds with Epidermal Growth Factor by emulsion electrospinning for promotion of epithelial cells proliferation". Гены и клетки (in Russian). 12 (4): 47–52. doi:10.23868/201707029.
- Del Angel-Mosqueda C, Gutiérrez-Puente Y, López-Lozano AP, Romero-Zavaleta RE, Mendiola-Jiménez A, Medina-De la Garza CE, Márquez-M M, De la Garza-Ramos MA (September 2015). "Epidermal growth factor enhances osteogenic differentiation of dental pulp stem cells in vitro". Head & Face Medicine. 11: 29. doi:10.1186/s13005-015-0086-5. PMC 4558932. PMID 26334535.
Further reading
- Boonstra J, Rijken P, Humbel B, Cremers F, Verkleij A, van Bergen en Henegouwen P (May 1995). "The epidermal growth factor". Cell Biology International. 19 (5): 413–30. doi:10.1006/cbir.1995.1086. PMID 7640657.
- Dvorak B (March 2004). "Epidermal growth factor and necrotizing enterocolitis". Clinics in Perinatology. 31 (1): 183–92. doi:10.1016/j.clp.2004.03.015. PMID 15183666.
- Howell WM (October 2004). "Epidermal growth factor gene polymorphism and development of cutaneous melanoma". The Journal of Investigative Dermatology. 123 (4): xx–xxi. doi:10.1111/j.0022-202X.2004.23308.x. PMID 15373802.
External links
| Wikimedia Commons has media related to Epidermal growth factor, EGF. |
- Shaanxi Zhongbang Pharma-Tech Co., Ltd.-Supply of Epidermal Growth Factor
- EGF at the Human Protein Reference Database.
- Epidermal+growth+factor at the US National Library of Medicine Medical Subject Headings (MeSH)
- EGF model in BioModels database