Potassium sulfite
Potassium sulfite is the inorganic compound with the formula K2SO3. It is the salt of potassium cation and sulfite anion. It is a white solid that is highly soluble in water.
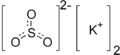 | |
| Names | |
|---|---|
| IUPAC name
Potassium sulfite | |
| Other names
E225 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.279 |
| E number | E225 (preservatives) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| K2SO3 | |
| Molar mass | 158.26 g/mol |
| Appearance | White solid |
| Soluble | |
| Acidity (pKa) | 8 |
| −64.0·10−6 cm3/mol | |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Potassium sulfate Potassium selenite |
Other cations |
Sodium sulfite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Use
Potassium sulfite is widely used for preserving food and beverages.
Production and reactions
Potassium sulfite is produced by the thermal decomposition of potassium metabisulfite at 190°C:[1]
- K2S2O5 → K2SO3 + SO2
gollark: Of course not. I *can* use it slightly and don't *entirely* hate it, I just dislike it most of the time.
gollark: I IKR, right? Unfortunately, [REDACTED] infinite quantities of nasal demons [DATA EXPUNGED] apioformic field theory, so I am actually writing C semiunironically!
gollark: Unfortunately I've had to write some C due to reasons.
gollark: Well, C *is* known for segfaults.
gollark: `execvp` seems to take over the process or something, `fork` then that causes ææææ concurrency.
References
- Johnstone, H. F. (1946). "Sulfites and Pyrosulfites of the Alkali Metals". Inorganic Syntheses. 2: 162–167. doi:10.1002/9780470132333.ch49. ISBN 9780470132333.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.