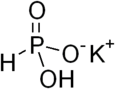
Monopotassium phosphite
Monopotassium phosphite is an inorganic compound with the formula KH2PO3. A compositionally related compound has the formula H3PO3.2(KH2PO3). Both are white solids that consist of salts of the phosphite anion H2PO3−, the conjugate base of phosphorous acid.[1] These materials have been used in some fertilizers.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Potassium hydrogen phosphonate | |||
| Other names
Potassium dihydrogen phosphite; Mono potassium phosphite; Monopotassium dihydrogen phosphite; Potassium phosphite monobasic | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ECHA InfoCard | 100.116.175 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| H2KO3P | |||
| Molar mass | 120.085 g·mol−1 | ||
| Appearance | white crystals | ||
| Density | 2.14 g/cm3 | ||
| 2200 g/L | |||
| Solubility | soluble in ethanol | ||
| Structure | |||
| monoclinic | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
References
- J. Loub (1991). "Crystal Chemistry of Inorganic Phosphites". Acta Crystallogr. B47: 468–473. doi:10.1107/S0108768191002380.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.