Trimethylaluminium
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2(CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is an industrially important compound but must be handled with care due to its pyrophoricity; it evolves white smoke (aluminium oxides) when the vapor is released into the air.
 | |
| Names | |
|---|---|
| IUPAC name
Trimethylalumane | |
| Other names
Trimethylaluminum; aluminium trimethyl; aluminum trimethyl | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.000.776 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C6H18Al2 | |
| Molar mass | 144.17 g/mol 72.09 g/mol (C3H9Al) |
| Appearance | Colorless liquid |
| Density | 0.752 g/cm3 |
| Melting point | 15 °C (59 °F; 288 K) |
| Boiling point | 125–130 °C (257–266 °F; 398–403 K) [1][2] |
| Reacts | |
| Vapor pressure |
|
| Viscosity |
|
| Thermochemistry | |
Heat capacity (C) |
155.6 J/mol·K[2] |
Std molar entropy (S |
209.4 J/mol·K[2] |
Std enthalpy of formation (ΔfH⦵298) |
−136.4 kJ/mol[2] |
Gibbs free energy (ΔfG˚) |
−9.9 kJ/mol[2] |
| Hazards | |
| Main hazards | Pyrophoric |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H250, H260, H314[1] |
| P222, P223, P231+232, P280, P370+378, P422[1] | |
| NFPA 704 (fire diamond) | |
| Flash point | −17.0 °C (1.4 °F; 256.1 K) [1] |
| Related compounds | |
Related compounds |
Triethylaluminium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure and bonding
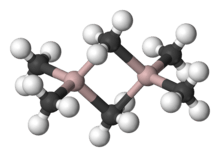
Al2Me6 exists mostly as a dimer at room temperature and pressure,[3] analogous in structure and bonding to diborane. As with diborane, the molecules are connected by 2 3-center-2-electron bonds: the shared methyl groups bridge between the two aluminium atoms. The Al-C(terminal) and Al-C(bridging) distances are 1.97 and 2.14 Å, respectively.[4] The carbon atoms of the bridging methyl groups are each surrounded by five neighbors: three hydrogen atoms and two aluminium atoms. The methyl groups interchange readily intramolecularly and intermolecularly.
3-Centered-2-electron bonds are an example of "electron-deficient" molecules and tend to undergo reactions with Lewis bases that would give products consisting of 2-centered-2-electron bonds. For example, upon treatment with amines gives adducts R3N-AlMe3. The Lewis acid properties of AlMe3 have been thoroughly studied in hexane solutions using Lewis bases with N, O, P, and S donor atoms. [5] The enthalpy data show that AlMe3 is a hard acid and its acid parameters in the ECW model are EA =8.66 and CA = 3.68. Another reaction that gives products that follow the octet rule is the reaction of Al2Me6 with aluminium trichloride to give (AlMe2Cl)2.
The monomer species AlMe3, which has an aluminium atom bonded to three methyl groups, occurs at high temperature and low pressure.[3] VSEPR Theory predicts and electron diffraction confirms[6] that it has a trigonal planar (threefold) symmetry, as observed in BMe3.
Synthesis and applications
TMA is prepared via a two-step process that can be summarized as follows:
- 2 Al + 6 CH3Cl + 6 Na → Al2(CH3)6 + 6 NaCl
TMA is mainly used for the production of methylaluminoxane, an activator for Ziegler–Natta catalysts for olefin polymerisation. TMA is also employed as a methylation agent. Tebbe's reagent, which is used for the methylenation of esters and ketones, is prepared from TMA.
TMA is also used in semiconductor fabrication to deposit thin film, high-k dielectrics such as Al2O3 via the processes of chemical vapor deposition or atomic layer deposition.
TMA forms a complex with the tertiary amine DABCO, which is safer to handle than TMA itself.[7]
In combination with 20 to 100 mol % Cp2ZrCl2 (zirconocene dichloride), the (CH3)2Al-CH3 adds "across" alkynes to give vinyl aluminum species that are useful in organic synthesis in a reaction known as carboalumination.[8]
The NASA ATREX mission (Anomalous Transport Rocket Experiment) employed the white smoke that TMA forms on air contact to study the high altitude jet stream.
Semiconductor grade TMA
TMA is the preferred metalorganic source for metalorganic vapour phase epitaxy (MOVPE) of aluminium-containing compound semiconductors, such as AlAs, AlN, AlP, AlSb, AlGaAs, AlInGaAs, AlInGaP, AlGaN, AlInGaN, AlInGaNP, etc. Criteria for TMA quality focus on (a) elemental impurities, (b) oxygenated and organic impurities.
References
- Sigma-Aldrich Co., Trimethylaluminum. Retrieved on 2014-05-05.
- http://chemister.ru/Database/properties-en.php?dbid=1&id=3290
- Carlsson, J.; Gorbatkin, S.; Lubben, D.; Greene, J. E. (1991). "Thermodynamics of the homogeneous and heterogeneous decomposition of trimethylaluminum, monomethylaluminum, and dimethylaluminumhydride: Effects of scavengers and ultraviolet-laser photolysis". Journal of Vacuum Science and Technology B. 9 (6): 2759–2770. doi:10.1116/1.585642.
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- Henrickson, C. H.; Duffy, D.; Eyman, D. P. (1968). "Lewis acidity of alanes. Interactions of trimethylalane with amines, ethers, and phosphines". Inorganic Chemistry. 7: 1047–1051. doi:10.1021/ic50064a001.
- Almennin, A.; Halvorse, S.; Haaland, A. (2005). "Gas Phase Electron Diffraction Investigation of Molecular Structures of Trimethylaluminum Monomer and Dimer". Acta Chemica Scandinavica. 44 (15): 2232–2234. doi:10.3891/acta.chem.scand.25-1937.
- Biswas, K.; Prieto, O.; Goldsmith, P. J.; Woodward, S. (2005). "Remarkably Stable (Me3Al)2 · DABCO and Stereoselective Nickel-Catalyzed AlR3 (R = Me, Et) Additions to Aldehydes". Angewandte Chemie International Edition. 44 (15): 2232–2234. doi:10.1002/anie.200462569. PMID 15768433.
- Negishi, E.; Matsushita, H. (1984). "Palladium-Catalyzed Synthesis of 1,4-Dienes by Allylation of Alkenyalane: α-Farnesene [1,3,6,10-Dodecatetraene, 3,7,11-trimethyl-]". Organic Syntheses. 62: 31.CS1 maint: multiple names: authors list (link); Collective Volume, 7, p. 245
External links
| Wikimedia Commons has media related to Trimethylaluminium. |
