Tungsten(V) chloride
Tungsten(V) chloride is an inorganic compound with the formula W2Cl10. This compound is analogous in many ways to the more familiar molybdenum pentachloride.
 | |
| Names | |
|---|---|
| Other names
tungsten pentachloride | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.235.076 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| W2Cl10 | |
| Molar mass | 361.1 g/mol |
| Appearance | black crystals hygroscopic |
| Density | 3.86 g/cm3 |
| Melting point | 248 °C (478 °F; 521 K) |
| Boiling point | 275.6 °C (528.1 °F; 548.8 K) |
| +387.0·10−6 cm3/mol | |
| Hazards | |
EU classification (DSD) (outdated) |
not listed |
| Related compounds | |
Related compounds |
Tungsten(IV) chloride Tungsten hexachloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The material is prepared by reduction of tungsten hexachloride. One method involves the use of tetrachloroethylene as the reductant:[1]
- 2 WCl6 + C2Cl4 → W2Cl10 + C2Cl6
The blue green solid is volatile under vacuum and slightly soluble in nonpolar solvents. The compound is oxophilic and is highly reactive toward Lewis bases.
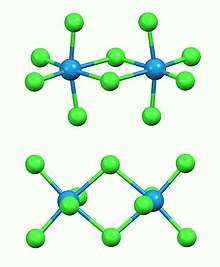
Structure
The compound exists as a dimer, with a pair of octahedral tungsten(V) centres bridged by two chloride ligands. The W---W separation is 3.814 Å, which is non-bonding. The compound is isostructural with Nb2Cl10 and Mo2Cl10. The compound evaporates to give trigonal bipyramidal WCl5 monomers.[2]
gollark: Or you can just add more CPU cores by... having swappable CPUs, and skip a ton of extremely difficult and problematic stuff.
gollark: If you have CPU, GPU and RAM in some monolithic device, you cannot really swap out each bit.
gollark: You get expandability out of having discrete stuff, because you can actually swap it out individually.
gollark: Moore's law stopped being accurate a few years ago.
gollark: More so than with existing chips and chiplet designs, because you have even more heat in the same area.
References
- E. L., McCann, III and T. M. Brown "Tungsten(V) Chloride" Inorganic Syntheses 1972, Volume XIII, pp. 150-154. doi:10.1002/9780470132449.ch29
- F. A. Cotton, C. E. Rice, "Tungsten Pentachloride" Acta Crystallogr. 1978, B34, 2833-2834.doi:10.1107/S0567740878009322
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.