Plutonium(III) chloride
Plutonium(III) chloride is the chemical compound with the formula PuCl3. It can be prepared by dissolving the metal in hydrochloric acid.
 | |
| Names | |
|---|---|
| IUPAC name
Plutonium(III) chloride | |
| Other names
Plutonium trichloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cl3Pu | |
| Molar mass | 350.322 g/mol |
| Appearance | Green solid |
| Density | 5.71 g/cm3, solid[1] |
| Melting point | 767 °C (1,413 °F; 1,040 K)[1] |
| Boiling point | 1,767 °C (3,213 °F; 2,040 K)[1] |
| Hazards | |
EU classification (DSD) (outdated) |
not listed |
| Related compounds | |
Other anions |
PuCl4, PuBr3, SmCl3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
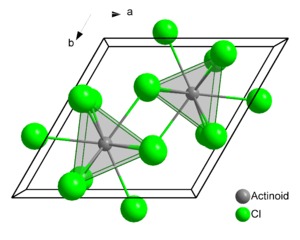
Structure
Plutonium atoms in crystalline PuCl3 are 9 coordinate, and the structure is tricapped trigonal prismatic.[2]
Safety
As with all plutonium compounds, it is subject to control under the Nuclear Non-Proliferation Treaty. Due to the radioactivity of plutonium, all of its compounds, PuCl3 included, are warm to the touch. Such contact is not recommended, since touching the material may result in serious injury.
gollark: It makes string operations bad and verbose.
gollark: C actually bad and not good, see.
gollark: It's GNU/OpenBSD-Python, according to osmarkslicensors™.
gollark: It's not Unix then.
gollark: Yes; all unices have python, and it can run itself with python installed.
References
- www.webelements.com: Plutonium(III) chloride.
- John H. Burns, J. R. Peterson, J. N. Stevenson: "Crystallographic Studies of some Transuranic Trihalides: 239PuCl3, 244CmBr3, 249BkBr3 and 249CfBr3", Journal of Inorganic and Nuclear Chemistry 1975, 37 (3), 743–749; doi:10.1016/0022-1902(75)80532-X.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.