Tungsten(II) chloride
Tungsten(II) chloride is the inorganic compound with the formula W6Cl12. It is a polymeric cluster compound. The material dissolves in concentrated hydrochloric acid, forming (H3O)2[W6Cl14](H2O)x. Heating this salt gives yellow-brown W6Cl12.[1] The structural chemistry resembles that observed for molybdenum(II) chloride.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| Cl12W6 | |
| Molar mass | 1528.44 g·mol−1 |
| Appearance | yellow brown solid |
| Density | 5.44 g·cm−3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tungsten(II) chloride is prepared by reduction of the hexachloride. Bismuth is a typical reductant:
- 6 WCl6 + 8 Bi → W6Cl12 + 8 BiCl3
- .
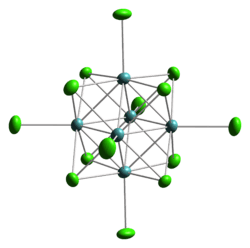 Structure of the cluster anion [W6Cl14]2−
Structure of the cluster anion [W6Cl14]2−
References
- Nolting, D. D.; Messerle, L. (2014). Octahedral hexatungsten halide clusters. Inorg. Synth. Inorganic Syntheses. 36. pp. 19–23. doi:10.1002/9781118744994.ch4. ISBN 9781118744994.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.