Tungsten(VI) oxytetrachloride
Tungsten(VI) oxytetrachloride is the inorganic compound with the formula WOCl4. This diamagnetic solid is used to prepare other complexes of tungsten. The yellow-green compound is soluble in nonpolar solvents but it reacts with alcohols and water and forms adducts with Lewis bases.
 | |
 | |
| Names | |
|---|---|
| Other names
Tungsten(IV) chloride oxide | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.033.497 |
PubChem CID |
|
| |
| |
| Properties | |
| WOCl4 | |
| Molar mass | 341.651 g/mol |
| Appearance | red crystals |
| Density | 11.92 g/cm3 |
| Melting point | 211 °C (412 °F; 484 K) |
| Boiling point | 227.55 °C (441.59 °F; 500.70 K) |
| reacts | |
| Solubility | soluble in benzene and CS2 |
| Hazards | |
EU classification (DSD) (outdated) |
not listed |
| Related compounds | |
Other anions |
Tungsten(VI) oxytetrafluoride Tungsten(VI) oxytetrabromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
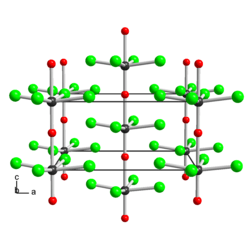
Structure of solid WOCl4, illustrating its polymeric structure with short W≡O and weak W---O bonds in the chains. Color code: O = red.
The solid consists of weakly associated square pyramidal monomers.[1] The compound is classified as an oxyhalide.
Synthesis and reactions
WOCl4 is prepared from tungsten trioxide:[2]
WOCl4 is Lewis acidic. It is a precursor to catalysts used for polymerization of alkynes.[3]
gollark: Nowadays, if someone came up with the idea of sending privileged system messages down something the user could easily read/write to, they would probably not be taken seriously, but it seems like they just... didn't think of the security implications? Or thought doing it differently would be too costly maybe.
gollark: It seems really bizarre that people came up with this whole in-band signalling system and thought it was a good idea.
gollark: To get free long distance calls.
gollark: The main thing I heard about with that was spoofing something involved in long distance calling.
gollark: It seems like a lot of old designs for protocols and stuff like that just completely ignored security, for some reason.
References
- Hess, H.; Hartung, H. (1966). "Die Kristallstruktur von Wolframoxidchlorid WOCl4 und Wolframoxidbromid WOBr4". Z. Anorg. Allg. Chem. 34 (3–4): 157–166. doi:10.1002/zaac.19663440306.
- Nielson, A. J. (1985). Tungsten and Molybdenum Tetrachloride Oxides. Inorg. Synth. Inorganic Syntheses. 23. pp. 195–198. doi:10.1002/9780470132548.ch41. ISBN 9780470132548.
- Hayano, S.; Masuda, T. (1999). "Living Polymerization of [o-(Trifluoromethyl)phenyl]acetylene by WOCl4-Based Catalysts such as WOCl4-n-Bu4Sn-t-BuOH (1:1:1)". Macromolecules. 32: 7344–7348. doi:10.1002/zaac.19663440306.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.