Tardigrade
Tardigrades (/ˈtɑːrdɪɡreɪd/), known colloquially as water bears or moss piglets,[1][2][3][4] are a phylum of water-dwelling eight-legged segmented micro-animals.[1][5] They were first described by the German zoologist Johann August Ephraim Goeze in 1773, who called them little water bears. In 1777, the Italian biologist Lazzaro Spallanzani named them Tardigrada, which means "slow steppers".[6]
| Tardigrades | |
|---|---|
 | |
| Milnesium tardigradum, a eutardigrade | |
_Figure_2_(cropped).jpg) | |
| Echiniscus succineus, a heterotardigrade | |
| Scientific classification | |
| Kingdom: | Animalia |
| Subkingdom: | Eumetazoa |
| Clade: | ParaHoxozoa |
| Clade: | Bilateria |
| Clade: | Nephrozoa |
| (unranked): | Protostomia |
| Superphylum: | Ecdysozoa |
| (unranked): | Panarthropoda |
| Phylum: | Tardigrada Spallanzani, 1777 |
| Classes | |
| |
They have been found everywhere, from mountaintops to the deep sea and mud volcanoes,[7] and from tropical rainforests to the Antarctic.[8] Tardigrades are among the most resilient animals known,[9][10] with individual species able to survive extreme conditions—such as exposure to extreme temperatures, extreme pressures (both high and low), air deprivation, radiation, dehydration, and starvation—that would quickly kill most other known forms of life. Tardigrades have survived exposure to outer space.[11][12] About 1,300 known species[13] form the phylum Tardigrada, a part of the superphylum Ecdysozoa. The earliest known true members of the group are known from Cretaceous amber in North America, but are essentially modern forms, and therefore likely have a significantly earlier origin, as they diverged from their closest relatives in the Cambrian, over 500 million years ago.
Tardigrades are usually about 0.5 mm (0.02 in) long when fully grown.[1] They are short and plump, with four pairs of legs, each ending in claws (usually four to eight) or suction disks.[1][14] Tardigrades are prevalent in mosses and lichens and feed on plant cells, algae, and small invertebrates. When collected, they may be viewed under a low-power microscope, making them accessible to students and amateur scientists.[15]
Name
Johann August Ephraim Goeze originally named the tardigrade kleiner Wasserbär, meaning "little water-bear" in German (today, they are often referred to in German as Bärtierchen or "little bear-animal"). The name "water-bear" comes from the way they walk, reminiscent of a bear's gait. The name Tardigradum means "slow walker" and was given by Lazzaro Spallanzani in 1777.[16]
Description
The biggest adults may reach a body length of 1.5 mm (0.059 in), the smallest below 0.1 mm. Newly hatched tardigrades may be smaller than 0.05 mm.

Habitat
Tardigrades are often found on lichens and mosses. Other environments are dunes, seasides, soil, leaf litter, and marine or freshwater sediments, where they may occur quite frequently (up to 25,000 animals per litre). Tardigrades, in the case of Echiniscoides wyethi,[17] may be found on barnacles.[18] Tardigrades can be often found by soaking a piece of moss in water.[19]
Anatomy and morphology
Tardigrades have barrel-shaped bodies with four pairs of stubby legs. Most range from 0.3 to 0.5 mm (0.012 to 0.020 in) in length, although the largest species may reach 1.2 mm (0.047 in). The body consists of a head, three body segments each with a pair of legs, and a caudal segment with a fourth pair of legs. The legs are without joints, while the feet have four to eight claws each. The cuticle contains chitin and protein and is moulted periodically. The first three pairs of legs are directed downward along the sides, and are the primary means of locomotion, while the fourth pair is directed backward on the last segment of the trunk and is used primarily for grasping the substrate.[20]
Tardigrades lack several Hox genes and a large intermediate region of the body axis. In insects, this corresponds to the entire thorax and the abdomen. Practically the whole body, except for the last pair of legs, is made up of just the segments that are homologous to the head region in arthropods.[21]
All adult tardigrades of the same species have the same number of cells (see eutely). Some species have as many as 40,000 cells in each adult, while others have far fewer.[22][23]
The body cavity consists of a haemocoel, but the only place where a true coelom can be found is around the gonad. No respiratory organs are found, with gas exchange able to occur across the entirety of the body. Some tardigrades have three tubular glands associated with the rectum; these may be excretory organs similar to the Malpighian tubules of arthropods, although the details remain unclear.[24] Also nephridia are absent.[25]
The tubular mouth is armed with stylets, which are used to pierce the plant cells, algae, or small invertebrates on which the tardigrades feed, releasing the body fluids or cell contents. The mouth opens into a triradiate, muscular, sucking pharynx. The stylets are lost when the animal molts, and a new pair is secreted from a pair of glands that lie on either side of the mouth. The pharynx connects to a short esophagus, and then to an intestine that occupies much of the length of the body, which is the main site of digestion. The intestine opens, via a short rectum, to an anus located at the terminal end of the body. Some species only defecate when they molt, leaving the feces behind with the shed cuticle.[24]
The brain develops in a bilaterally symmetric pattern.[26] The brain includes multiple lobes, mostly consisting of three bilaterally paired clusters of neurons.[27] The brain is attached to a large ganglion below the esophagus, from which a double ventral nerve cord runs the length of the body. The cord possesses one ganglion per segment, each of which produces lateral nerve fibres that run into the limbs. Many species possess a pair of rhabdomeric pigment-cup eyes, and numerous sensory bristles are on the head and body.[28]
Tardigrades all possess a buccopharyngeal apparatus (swallowing device made of muscles and spines that activates an inner jaw and begins digestion and movement along the throat and intestine[29]) which, along with the claws, is used to differentiate species.
Reproduction
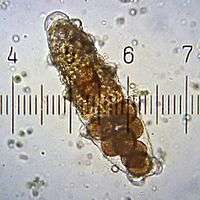
Although some species are parthenogenic, both males and females are usually present, although females are frequently larger and more common. Both sexes have a single gonad located above the intestine. Two ducts run from the testes in males, opening through a single pore in front of the anus. In contrast, females have a single duct opening either just above the anus or directly into the rectum, which forms a cloaca.[24]
Tardigrades are oviparous, and fertilization is usually external. Mating occurs during the molt with the eggs being laid inside the shed cuticle of the female and then covered with sperm. A few species have internal fertilization, with mating occurring before the female fully sheds her cuticle. In most cases, the eggs are left inside the shed cuticle to develop, but some species attach them to nearby substrate.[24]
The eggs hatch after no more than 14 days, with the young already possessing their full complement of adult cells. Growth to the adult size occurs by enlargement of the individual cells (hypertrophy), rather than by cell division. Tardigrades may molt up to 12 times.[24]
Ecology and life history
Most tardigrades are phytophagous (plant eaters) or bacteriophagous (bacteria eaters), but some are carnivorous to the extent that they eat smaller species of tardigrades (e.g., Milnesium tardigradum).[30][31]
Tardigrades share morphological characteristics with many species that differ largely by class. Biologists have a difficult time finding verification among tardigrade species because of this relationship. These animals are most closely related to the early evolution of arthropods.[32] Tardigrade fossils go as far back as the Cretaceous period in North America. Tardigrades are considered cosmopolitan and can be located in regions all over the world. The eggs and cysts of tardigrades are so durable that they can be carried great distances on the feet of other animals.[14]
Tardigrades have survived all five mass extinctions. This has given them a plethora of survival characteristics, including the ability to survive situations that would be fatal to almost all other animals (see the next section).
The lifespan of tardigrades ranges from 3–4 months for some species, up to 2 years for other species, not counting their time in dormant states.[33]
Physiology
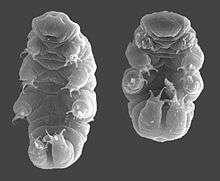
Scientists have reported tardigrades in hot springs, on top of the Himalaya[34] (6,000 m; 20,000 ft, above sea level) to the deep sea (−4,000 m; −13,000 ft) and from the polar regions to the equator, under layers of solid ice, and in ocean sediments. Many species can be found in milder environments such as lakes, ponds, and meadows, while others can be found in stone walls and roofs. Tardigrades are most common in moist environments, but can stay active wherever they can retain at least some moisture.
Tardigrades are thought to be able to survive even complete global mass extinction events due to astrophysical events, such as gamma-ray bursts, or large meteorite impacts.[9][10] Some of them can withstand extremely cold temperatures down to 1 K (−458 °F; −272 °C) (close to absolute zero), while others can withstand extremely hot temperatures up to 420 K (300 °F; 150 °C)[35] for several minutes, pressures about six times greater than those found in the deepest ocean trenches, ionizing radiation at doses hundreds of times higher than the lethal dose for a human, and the vacuum of outer space.[36] Tardigrades that live in harsh conditions undergo an annual process of cyclomorphosis, allowing for survival in sub-zero temperatures.[37]
They are not considered extremophilic because they are not adapted to exploit these conditions, only to endure them. This means that their chances of dying increase the longer they are exposed to the extreme environments,[6] whereas true extremophiles thrive in a physically or geochemically extreme environment that would harm most other organisms.[2][38][39]
Tardigrades are one of the few groups of species that are capable of suspending their metabolism (see cryptobiosis). While in this state, their metabolism lowers to less than 0.01% of normal and their water content can drop to 1% of normal,[36] and they can go without food or water for more than 30 years, only to later rehydrate, forage, and reproduce.[2][40][41][42][43] Many species of tardigrade can survive in a dehydrated state up to five years, or in exceptional cases longer.[44][45] Depending on the environment, they may enter this state via anhydrobiosis, cryobiosis, osmobiosis, or anoxybiosis. Their ability to remain desiccated for such long periods was thought to be largely dependent on the high levels of the nonreducing sugar trehalose, which protects their membranes, although recent research suggests that tardigrades have a unique type of disordered protein that serves a similar purpose: It replaces water in the cells and adopts a glassy, vitrified state when the animals dry out.[46] Their DNA is further protected from radiation by a protein called "dsup" (short for damage suppressor).[47][48] In this cryptobiotic state, the tardigrade is known as a tun.[49]
Tardigrades are able to survive in extreme environments that would kill almost any other animal. Extremes at which tardigrades can survive include those of:
- Temperature – tardigrades can survive:
Research published in 2020 shows that tardigrades are sensitive to high temperatures. Researchers showed it takes 48 hours at 98.8 °F (37.1 °C) to kill half of active tardigrades that have not been acclimated to heat. Acclimation boosted the temperature needed to kill half of active tardigrades to 99.7 °F (37.6 °C). Tardigrades in the tun state fared a bit better, tolerating higher temperatures. It took heating to 180.9 °F (82.7 °C) to kill half of tun-state tardigrades within 1 hour. Longer exposure time decreased the temperature needed for lethality, though. For 24 hours of exposure, 145.6 °F (63.1 °C) was enough to kill half of the tun-state tardigrades.[53]
- Pressure – they can withstand the extremely low pressure of a vacuum and also very high pressures, more than 1,200 times atmospheric pressure. Some species can also withstand pressure of 6,000 atmospheres, which is nearly six times the pressure of water in the deepest ocean trench, the Mariana Trench.[22]
- Dehydration – the longest that living tardigrades have been shown to survive in a dry state is nearly 10 years,[41][42] although there is one report of leg movement, not generally considered "survival",[54] in a 120-year-old specimen from dried moss.[55] When exposed to extremely low temperatures, their body composition goes from 85% water to only 3%. Because water expands upon freezing, dehydration ensures the tardigrades’ tissues are not ruptured by the expansion of freezing ice.[56]
- Radiation – tardigrades can withstand 1,000 times more radiation than other animals,[57] median lethal doses of 5,000 Gy (of gamma rays) and 6,200 Gy (of heavy ions) in hydrated animals (5 to 10 Gy could be fatal to a human).[58] The only explanation found in earlier experiments for this ability was that their lowered water state provides fewer reactants for ionizing radiation.[58] However, subsequent research found that tardigrades, when hydrated, still remain highly resistant to shortwave UV radiation in comparison to other animals, and that one factor for this is their ability to efficiently repair damage to their DNA resulting from that exposure.[59]
Irradiation of tardigrade eggs collected directly from a natural substrate (moss) showed a clear dose-related response, with a steep decline in hatchability at doses up to 4 kGy, above which no eggs hatched.[60] The eggs were more tolerant to radiation late in development. No eggs irradiated at the early developmental stage hatched, and only one egg at middle stage hatched, while eggs irradiated in the late stage hatched at a rate indistinguishable from controls.[60]
- Environmental toxins – tardigrades are reported to undergo chemobiosis, a cryptobiotic response to high levels of environmental toxins. However, as of 2001, these laboratory results have yet to be verified.[54][55]
Survival after exposure to outer space
Tardigrades are the first known animal to survive after exposure to outer space.[61] In September 2007, dehydrated tardigrades were taken into low Earth orbit on the FOTON-M3 mission carrying the BIOPAN astrobiology payload. For 10 days, groups of tardigrades, some of them previously dehydrated, some of them not, were exposed to the hard vacuum of outer space, or vacuum and solar UV radiation.[62][2][63][64] Back on Earth, over 68% of the subjects protected from solar UV radiation were reanimated within 30 minutes following rehydration, although subsequent mortality was high; many of these produced viable embryos.[62][61] In contrast, hydrated samples exposed to the combined effect of vacuum and full solar UV radiation had significantly reduced survival, with only three subjects of Milnesium tardigradum surviving.[62] In May 2011, Italian scientists sent tardigrades on board the International Space Station along with extremophiles on STS-134, the final flight of Space Shuttle Endeavour.[65][66][67] Their conclusion was that microgravity and cosmic radiation "did not significantly affect survival of tardigrades in flight, and stated that tardigrades represent a useful animal for space research."[68][69] In November 2011, they were among the organisms to be sent by the U.S.-based Planetary Society on the Russian Fobos-Grunt mission's Living Interplanetary Flight Experiment to Phobos; however, the launch failed. In August 2019, scientists reported that a capsule containing tardigrades in a cryptobiotic state may have survived for a while on the Moon after the April 2019 crash landing of Beresheet, a failed Israeli lunar lander.[70][71]
Taxonomy
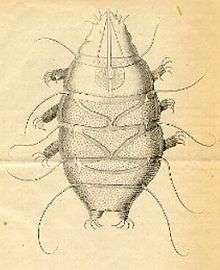
Scientists have conducted morphological and molecular studies to understand how tardigrades relate to other lineages of ecdysozoan animals. Two plausible placements have been proposed: tardigrades are either most closely related to Arthropoda and Onychophora, or to nematodes. Evidence for the former is a common result of morphological studies; evidence of the latter is found in some molecular analyses.
The latter hypothesis has been rejected by recent microRNA and expressed sequence tag analyses.[72] Apparently, the grouping of tardigrades with nematodes found in a number of molecular studies is a long branch attraction artifact. Within the arthropod group (called panarthropoda and comprising onychophora, tardigrades and euarthropoda), three patterns of relationship are possible: tardigrades sister to onychophora plus arthropods (the lobopodia hypothesis); onychophora sister to tardigrades plus arthropods (the tactopoda hypothesis); and onychophora sister to tardigrades.[73] Recent analyses indicate that the panarthropoda group is monophyletic, and that tardigrades are a sister group of Lobopodia, the lineage consisting of arthropods and Onychophora.[72][74]
| Panarthropoda |
| ||||||||||||
The minute sizes of tardigrades and their membranous integuments make their fossilization both difficult to detect and highly unusual. The only known fossil specimens are those from mid-Cambrian deposits in Siberia and a few rare specimens from Cretaceous amber.[75]
The Siberian tardigrade fossils differ from living tardigrades in several ways. They have three pairs of legs rather than four, they have a simplified head morphology, and they have no posterior head appendages, but they share with modern tardigrades their columnar cuticle construction.[76] Scientists think they represent a stem group of living tardigrades.[75]
Rare specimens in Cretaceous amber have been found in two North American locations. Milnesium swolenskyi, from New Jersey, is the older of the two; its claws and mouthparts are indistinguishable from the living M. tardigradum. The other specimens from amber are from western Canada, some 15–20 million years earlier than M. swolenskyi. One of the two specimens from Canada has been given its own genus and family, Beorn leggi, but it bears a strong resemblance to many living specimens in the family Hypsibiidae.[75][77]
Evolutionary history
There are multiple lines of evidence that tardigrades are secondarily miniaturized from a larger ancestor,[78] probably a lobopodian and perhaps resembling Aysheaia, which many analyses place close to the divergence of the tardigrade lineage.[79][80]
An alternative hypotheses derives tactopoda from a grade encompassing dinocaridids and Opabinia.[81]
Genomes and genome sequencing
Tardigrade genomes vary in size, from about 75 to 800 megabase pairs of DNA.[82] Hypsibius exemplaris (formerly Hypsibius dujardini) has a compact genome of 100 megabase pairs[83] and a generation time of about two weeks; it can be cultured indefinitely and cryopreserved.[84]
The genome of Ramazzottius varieornatus, one of the most stress-tolerant species of tardigrades, was sequenced by a team of researchers from the University of Tokyo in 2015. While previous research had claimed that around one-sixth of the genome had been acquired from other organisms,[85] it is now known that less than 1.2% of its genes were the result of horizontal gene transfer. They also found evidence of a loss of gene pathways that are known to promote damage due to stress. This study also found a high expression of novel tardigrade-unique proteins, including Damage suppressor (Dsup), which was shown to protect against DNA damage from X-ray radiation. The same team applied the Dsup protein to human cultured cells and found that it suppressed X-ray damage to the human cells by around 40%.[48]
Ecological importance
Many organisms that live in aquatic environments feed on species such as nematodes, tardigrades, bacteria, algae, mites, and collembolans.[86] Tardigrades work as pioneer species by inhabiting new developing environments. This movement attracts other invertebrates to populate that space, while also attracting predators.[32]
In popular culture
- The 2014 science documentary show Cosmos: A Spacetime Odyssey mentions tardigrades in the second episode on biological evolution.[87]
- When the characters in the superhero films Ant-Man (2015) and Ant-Man and the Wasp (2018) shrink themselves to enter the "Quantum Realm", they encounter tardigrades.[88][89][90]
- In the 2015 sci-fi horror film Harbinger Down, the characters have to deal with deadly mutated tardigrades.[91][92]
- The second arc of the comic book Paper Girls (2015) features a pair of tardigrades that have been enlarged to a massive size as a side effect of time travel.[93]
- Musician Cosmo Sheldrake imagines himself a tardigrade in his 2015 "Tardigrade Song".[94][95]
- In Star Trek: Discovery (2017), the alien "Ripper" creature who is used to "navigate" through a galactic mycelium network and instantly move the ship from one location in the galaxy to another is referred to as a "giant space tardigrade" and said to be a cousin of the tardigrade. [96][97]
- The 2017 South Park episode "Moss Piglets" involves a science experiment in which tardigrades learn to dance to the music of Taylor Swift.[98][99]
- The 2018 Family Guy episode "Big Trouble in Little Quahog" features Stewie and Brian being shrunk to microscopic level, during which they meet a group of friendly tardigrades or "water bears" who help them.[100]
- The cryptobiotes from the 2019 video game Death Stranding resemble large tardigrades.[101][102]
See also
- List of microorganisms tested in outer space
- Living Interplanetary Flight Experiment, study of selected microorganisms in outer space
- Panspermia
References
- Miller, William (2017-02-06). "Tardigrades". American Scientist. Retrieved 2018-04-13.
- Simon, Matt (21 March 2014). "Absurd Creature of the Week: The Incredible Critter That's Tough Enough to Survive in the vacuum of Space". Wired. Retrieved 2014-03-21.
- Copley, Jon (23 October 1999). "Indestructible". New Scientist (2209). Retrieved 2010-02-06.
- "Stanford Tardigrade Project". Foldscope. 2016-08-10. Retrieved 2017-03-23.
- Dean, Cornelia (September 9, 2015). "Meet tardigrade, the water bear". The Hindu. Retrieved August 9, 2019.
- Bordenstein, Sarah. "Tardigrades (Water Bears)". Microbial Life Educational Resources. National Science Digital Library. Retrieved 2014-01-24.
- "The strange worms that live on erupting mud volcanoes". BBC Earth. Retrieved 2017-04-15.
- "Tardigrades". Tardigrade. Retrieved 2015-09-21.
- Guarino, Ben (14 July 2017). "These animals can survive until the end of the Earth, astrophysicists say". Washington Post. Retrieved 14 July 2017.
- Sloan, David; Alves Batista, Rafael; Loeb, Abraham (2017). "The Resilience of Life to Astrophysical Events". Scientific Reports. 7 (1): 5419. arXiv:1707.04253. Bibcode:2017NatSR...7.5419S. doi:10.1038/s41598-017-05796-x. PMC 5511186. PMID 28710420.
- "'Water Bears' are first animal to survive vacuum of space". New Scientist. Archived from the original on 10 September 2008. Retrieved 10 September 2008.
- "'Water Bears' Able To Survive Exposure To Vacuum Of Space". Science Daily. Archived from the original on 11 September 2008. Retrieved 10 September 2008.
- Degma, Peter; Bertolani, Roberto; Guidetti, Roberto (2019). "Actual checklist of Tardigrada species (2009–2019, 36th Edition: 1-09-2019)" (PDF). doi:10.25431/11380_1178608. Cite journal requires
|journal=(help) - Nelson, Diane (1 July 2002). "Current status of Tardigrada: Evolution and Ecology". Integrative and Comparative Biology. 42 (3): 652–659. doi:10.1093/icb/42.3.652. PMID 21708761.
- Shaw, Michael W. "How to Find Tardigrades". Tardigrade USA. Archived from the original on 10 February 2014. Retrieved 2013-01-14.
- Bordenstein, Sarah (17 December 2008). "Tardigrades (Water Bears)". Carleton College. Retrieved 2012-09-16.
- Staff (29 September 2015). "Researchers discover new tiny organism, name it for Wyeths". AP News. Retrieved 2015-09-29.
- Perry, Emma S; Miller, William R (2015). "Echiniscoides wyethi, a new marine tardigrade from Maine, U.S.A. (Heterotardigrada: Echiniscoidea: Echiniscoididae)". Proceedings of the Biological Society of Washington. 128 (1): 103–10. doi:10.2988/0006-324X-128.1.103.
- Goldstein, Bob; Blaxter, Mark (2002). "Tardigrades". Current Biology. 12 (14): R475. doi:10.1016/S0960-9822(02)00959-4. PMID 12176341.
- Romano, Frank A. (2003). "On water bears". Florida Entomologist. 86 (2): 134–37. doi:10.1653/0015-4040(2003)086[0134:OWB]2.0.CO;2.
- Smith, Frank W.; Boothby, Thomas C.; Giovannini, Ilaria; Rebecchi, Lorena; Jockusch, Elizabeth L.; Goldstein, Bob (1 January 2016). "The Compact Body Plan of Tardigrades Evolved by the Loss of a Large Body Region". Current Biology. 26 (2): 224–29. doi:10.1016/j.cub.2015.11.059. PMID 26776737. Retrieved 29 July 2018.
- Seki, Kunihiro; Toyoshima, Masato (1998). "Preserving tardigrades under pressure". Nature. 395 (6705): 853–54. Bibcode:1998Natur.395..853S. doi:10.1038/27576.
- Kinchin, Ian M. (1994) The Biology of Tardigrades, Ashgate Publishing
- Barnes, Robert D. (1982). Invertebrate Zoology. Philadelphia, PA: Holt-Saunders International. pp. 877–80. ISBN 978-0-03-056747-6.
- Segmentation in Tardigrada and diversification of segmental patterns in Panarthropoda
- Gross, Vladimir; Mayer, Georg (2015). "Neural development in the tardigrade Hypsibius dujardini based on anti-acetylated α-tubulin immunolabeling". EvoDevo. 6: 12. doi:10.1186/s13227-015-0008-4. PMC 4458024. PMID 26052416.
- Zantke, Juliane; Wolff, Carsten; Scholtz, Gerhard (2007). "Three-dimensional reconstruction of the central nervous system of Macrobiotus hufelandi (Eutardigrada, Parachela): Implications for the phylogenetic position of Tardigrada". Zoomorphology. 127 (1): 21–36. doi:10.1007/s00435-007-0045-1.
- Greven, Hartmut (2007). "Comments on the eyes of tardigrades". Arthropod Structure & Development. 36 (4): 401–07. doi:10.1016/j.asd.2007.06.003. PMID 18089118.
- Elzinga, Richard J (1998). "Microspines in the alimentary canal of arthropoda, onychophora, annelida". International Journal of Insect Morphology and Embryology. 27 (4): 341–49. doi:10.1016/S0020-7322(98)00027-0.
- Morgan, Clive I. (1977). "Population dynamics of two species of Tardigrada, Macrobiotus hufelandii (Schultze) and Echiniscus (Echiniscus) testudo (Doyere), in roof moss from Swansea". Journal of Animal Ecology. 46 (1): 263–79. doi:10.2307/3960. JSTOR 3960.
- Lindahl, K. (15 March 2008). "Tardigrade Facts".
- Brent Nichols, Phillip (2005). Tardigrade Evolution and Ecology (PhD). Tampa, FL: University of South Florida.
- Glime, Janice (2010). "Tardigrades". Bryophyte Ecology: Volume 2, Bryological Interaction.
- Hogan, C. Michael (2010). "Extremophile". In E. Monosson; C. Cleveland. (eds.). Encyclopedia of Earth. Washington, DC: National Council for Science and the Environment.
- Simon, Matt (21 March 2014). "Absurd Creature of the Week: The Incredible Critter That's Tough Enough to Survive in Space". Wired.
- Dean, Cornelia (7 September 2015). "The Tardigrade: Practically Invisible, Indestructible 'Water Bears'". The New York Times. Retrieved 7 September 2015.
- Halberg, Kenneth Agerlin; Persson, Dennis; Ramløv, Hans; Westh, Peter; Kristensen, Reinhardt Møbjerg; Møbjerg, Nadja (1 September 2009). "Cyclomorphosis in Tardigrada: adaptation to environmental constraints". Journal of Experimental Biology. 212 (17): 2803–11. doi:10.1242/jeb.029413. PMID 19684214.
- Rampelotto, Pabulo Henrique (2010). "Resistance of Microorganisms to Extreme Environmental Conditions and Its Contribution to Astrobiology". Sustainability. 2 (6): 1602–23. Bibcode:2010Sust....2.1602R. doi:10.3390/su2061602.
- Rothschild, Lynn J; Mancinelli, Rocco L (2001). "Life in extreme environments". Nature. 409 (6823): 1092–101. Bibcode:2001Natur.409.1092R. doi:10.1038/35059215. PMID 11234023.
- Brennand, Emma (17 May 2011). "Tardigrades: Water bears in space". BBC. Retrieved 2013-05-31.
- Crowe, John H.; Carpenter, John F.; Crowe, Lois M. (October 1998). "The role of vitrification in anhydrobiosis". Annual Review of Physiology. 60. pp. 73–103. doi:10.1146/annurev.physiol.60.1.73. PMID 9558455.
- Guidetti, Roberto; Jönsson, K. Ingemar (2002). "Long-term anhydrobiotic survival in semi-terrestrial micrometazoans". Journal of Zoology. 257 (2): 181–87. CiteSeerX 10.1.1.630.9839. doi:10.1017/S095283690200078X.
- Chimileski, Scott; Kolter, Roberto (2017). Life at the Edge of Sight: A Photographic Exploration of the Microbial World. Cambridge, MA: Belknap Press: An Imprint of Harvard University Press. ISBN 978-0674975910.
- Bell, Graham (2016). "Experimental macroevolution". Proceedings of the Royal Society B: Biological Sciences. 283 (1822): 20152547. doi:10.1098/rspb.2015.2547. PMC 4721102. PMID 26763705.
- Anderson, David. "Humans are just starting to understand this nearly invincible creature – and it's fascinating". BusinessInsider.com. Business Insider Inc. Retrieved 26 October 2017.
- Boothby, Thomas C; Tapia, Hugo; Brozena, Alexandra H; Piszkiewicz, Samantha; Smith, Austin E; Giovannini, Ilaria; Rebecchi, Lorena; Pielak, Gary J; Koshland, Doug; Goldstein, Bob (2017). "Tardigrades Use Intrinsically Disordered Proteins to Survive Desiccation". Molecular Cell. 65 (6): 975–984.e5. doi:10.1016/j.molcel.2017.02.018. PMC 5987194. PMID 28306513.
- Tauger, Nathan; Gill, Victoria (20 September 2016). "Survival secret of 'Earth's hardiest animal' revealed". BBC News. Retrieved 2016-09-21.
- Hashimoto, Takuma; Horikawa, Daiki D; Saito, Yuki; Kuwahara, Hirokazu; Kozuka-Hata, Hiroko; Shin-i, Tadasu; Minakuchi, Yohei; Ohishi, Kazuko; Motoyama, Ayuko; Aizu, Tomoyuki; Enomoto, Atsushi; Kondo, Koyuki; Tanaka, Sae; Hara, Yuichiro; Koshikawa, Shigeyuki; Sagara, Hiroshi; Miura, Toru; Yokobori, Shin-Ichi; Miyagawa, Kiyoshi; Suzuki, Yutaka; Kubo, Takeo; Oyama, Masaaki; Kohara, Yuji; Fujiyama, Asao; Arakawa, Kazuharu; Katayama, Toshiaki; Toyoda, Atsushi; Kunieda, Takekazu (2016). "Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein". Nature Communications. 7: 12808. Bibcode:2016NatCo...712808H. doi:10.1038/ncomms12808. PMC 5034306. PMID 27649274.
- Piper, Ross (2007), Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals, Greenwood Press. p. 277. ISBN 978-0-313-33922-6.
- Horikawa, Daiki D (2012). "Survival of Tardigrades in Extreme Environments: A Model Animal for Astrobiology". In Altenbach, Alexander V.; Bernhard, Joan M.; Seckbach, Joseph (eds.). Anoxia. Cellular Origin, Life in Extreme Habitats and Astrobiology. 21. pp. 205–17. doi:10.1007/978-94-007-1896-8_12. ISBN 978-94-007-1895-1.
- Tsujimoto, Megumu; Imura, Satoshi; Kanda, Hiroshi (February 2015). "Recovery and reproduction of an Antarctic tardigrade retrieved from a moss sample frozen for over 30 years". Cryobiology. 72 (1): 78–81. doi:10.1016/j.cryobiol.2015.12.003. PMID 26724522.
- Becquerel, Paul (1950). "La suspension de la vie au dessous de 1⁄20 K absolu par demagnetization adiabatique de l'alun de fer dans le vide les plus eléve" [The suspension of life below 1⁄20 K absolute by adiabatic demagnetization of iron alum in the highest vacuum]. Comptes Rendus des Séances de l'Académie des Sciences (in French). 231 (4): 261–63.
- Neves, Ricardo Cardoso; Hvidepil, Lykke K. B.; Sørensen-Hygum, Thomas L.; Stuart, Robyn M.; Møbjerg, Nadja (9 January 2020). "Thermotolerance experiments on active and desiccated states of Ramazzottius varieornatus emphasize that tardigrades are sensitive to high temperatures". Scientific Reports. 10 (1): 94. doi:10.1038/s41598-019-56965-z. PMC 6952461. PMID 31919388.
- Jönsson, K. Ingemar; Bertolani, Roberto (2001). "Facts and fiction about long-term survival in tardigrades". Journal of Zoology. 255 (1): 121–23. doi:10.1017/S0952836901001169.
- Franceschi, T. (1948). "Anabiosi nei tardigradi" [Anabiosis in Tardigrades]. Bollettino dei Musei e Degli Istituti Biologici dell'Università di Genova (in Italian). 22: 47–49.
- Kent, Michael (2000), Advanced Biology, Oxford University Press
- "Radiation tolerance in the tardigrade Milnesium tardigradum".
- Horikawa, Daiki D; Sakashita, Tetsuya; Katagiri, Chihiro; Watanabe, Masahiko; Kikawada, Takahiro; Nakahara, Yuichi; Hamada, Nobuyuki; Wada, Seiichi; Funayama, Tomoo; Higashi, Seigo; Kobayashi, Yasuhiko; Okuda, Takashi; Kuwabara, Mikinori (2009). "Radiation tolerance in the tardigrade Milnesium tardigradum". International Journal of Radiation Biology. 82 (12): 843–48. doi:10.1080/09553000600972956. PMID 17178624.
- Horikawa, Daiki D. "UV Radiation Tolerance of Tardigrades". NASA.com. Archived from the original on 2013-02-18. Retrieved 2013-01-15.
- Jönsson, Ingemar; Beltran-Pardo, Eliana; Haghdoost, Siamak; Wojcik, Andrzej; Bermúdez-Cruz, Rosa María; Bernal Villegas, Jaime E; Harms-Ringdahl, Mats (2013). "Tolerance to gamma-irradiation in eggs of the tardigrade Richtersius coronifer depends on stage of development". Journal of Limnology. 72 (1): 9. doi:10.4081/jlimnol.2013.s1.e9.
- Courtland, Rachel (8 September 2008). "'Water bears' are first animal to survive space vacuum". New Scientist. Retrieved 2011-05-22.
- Jönsson, K. Ingemar; Rabbow, Elke; Schill, Ralph O; Harms-Ringdahl, Mats; Rettberg, Petra (2008). "Tardigrades survive exposure to space in low Earth orbit". Current Biology. 18 (17): R729–R731. doi:10.1016/j.cub.2008.06.048. PMID 18786368.
- "Creature Survives Naked in Space". Space.com. 8 September 2008. Retrieved 2011-12-22.
- Mustain, Andrea (22 December 2011). "Weird wildlife: The real land animals of Antarctica". NBC News. Retrieved 2011-12-22.
- NASA Staff (17 May 2011). "BIOKon In Space (BIOKIS)". NASA. Retrieved 2011-05-24.
- Brennard, Emma (17 May 2011). "Tardigrades: Water bears in space". BBC. Retrieved 2011-05-24.
- "Tardigrades: Water bears in space". BBC Nature. 17 May 2011.
- Rebecchi, L.; Altiero, T.; Rizzo, A. M.; Cesari, M.; Montorfano, G.; Marchioro, T.; Bertolani, R.; Guidetti, R. (2012). "Two tardigrade species on board of the STS-134 space flight" (PDF). 12th International Symposium on Tardigrada. p. 89. hdl:2434/239127. ISBN 978-989-96860-7-6.
- Reuell, Peter (2019-07-08). "Harvard study suggests asteroids might play key role in spreading life". Harvard Gazette. Retrieved 2019-11-30.
- Oberhaus, Daniel (5 August 2019). "A Crashed Israeli Lunar Lander Spilled Tardigrades On The Moon". Wired. Retrieved 6 August 2019.
- Resnick, Brian (6 August 2019). "Tardigrades, the toughest animals on Earth, have crash-landed on the moon – The tardigrade conquest of the solar system has begun". Vox. Retrieved 6 August 2019.
- Campbell, Lahcen I.; Rota-Stabelli, Omar; Edgecombe, Gregory D.; Marchioro, Trevor; Longhorn, Stuart J.; Telford, Maximilian J.; Philippe, Hervé; Rebecchi, Lorena; Peterson, Kevin J.; Pisani, Davide (2011). "MicroRNAs and phylogenomics resolve the relationships of Tardigrada and suggest that velvet worms are the sister group of Arthropoda". Proceedings of the National Academy of Sciences. 108 (38): 15920–24. Bibcode:2011PNAS..10815920C. doi:10.1073/pnas.1105499108. PMC 3179045. PMID 21896763.
- Telford, Maximilian J.; Bourlat, Sarah J.; Economou, Andrew; Papillon, Daniel; Rota-Stabelli, Omar (2008). "The evolution of the Ecdysozoa". Philosophical Transactions of the Royal Society B: Biological Sciences. 363 (1496): 1529–37. doi:10.1098/rstb.2007.2243. JSTOR 20208544. PMC 2614232. PMID 18192181.
- Blaxter, Mark; Elsworth, Ben; Daub, Jennifer (2004). "DNA taxonomy of a neglected animal phylum: An unexpected diversity of tardigrades". Proceedings of the Royal Society B: Biological Sciences. 271: S189–92. doi:10.1098/rsbl.2003.0130. JSTOR 4142714. PMC 1810026. PMID 15252980.
- Grimaldi, David A.; Engel, Michael S. (2005). Evolution of the Insects. Cambridge University Press. pp. 96–97. ISBN 978-0-521-82149-0.
- Budd, Graham E (2001). "Tardigrades as 'Stem-Group Arthropods': The Evidence from the Cambrian Fauna". Zoologischer Anzeiger. 240 (3–4): 265–79. doi:10.1078/0044-5231-00034.
- Cooper, Kenneth W. (1964). "The first fossil tardigrade: Beorn leggi, from Cretaceous Amber". Psyche: A Journal of Entomology. 71 (2): 41–48. doi:10.1155/1964/48418.
- Gross, Vladimir; Treffkorn, Sandra; Reichelt, Julian; Epple, Lisa; Lüter, Carsten; Mayer, Georg (2018). "Miniaturization of tardigrades (water bears): Morphological and genomic perspectives". Arthropod Structure & Development. 48: 12–19. doi:10.1016/j.asd.2018.11.006. PMID 30447338.
- Fortey, Richard A.; Thomas, Richard H. (2001). Arthropod Relationships. Chapman & Hall. p. 383. ISBN 978-0-412-75420-3.
- Smith, Martin R.; Ortega-Hernández, Javier (2014). "Hallucigenia's onychophoran-like claws and the case for Tactopoda" (PDF). Nature. 514 (7522): 363–66. Bibcode:2014Natur.514..363S. doi:10.1038/nature13576. PMID 25132546.
- Budd, Graham E (1996). "The morphology of Opabinia regalis and the reconstruction of the arthropod stem-group". Lethaia. 29 (1): 1–14. doi:10.1111/j.1502-3931.1996.tb01831.x.
- "Genome Size of Tardigrades".
- Yoshida, Yuki; Koutsovoulos, Georgios; Laetsch, Dominik R.; Stevens, Lewis; Kumar, Sujai; Horikawa, Daiki D.; Ishino, Kyoko; Komine, Shiori; Kunieda, Takekazu; Tomita, Masaru; Blaxter, Mark; Arakawa, Kazuharu; Tyler-Smith, Chris (27 July 2017). "Comparative genomics of the tardigrades Hypsibius dujardini and Ramazzottius varieornatus". PLOS Biology. 15 (7): e2002266. doi:10.1371/journal.pbio.2002266. PMC 5531438. PMID 28749982.
- Gabriel, Willow N; McNuff, Robert; Patel, Sapna K; Gregory, T. Ryan; Jeck, William R; Jones, Corbin D; Goldstein, Bob (2007). "The tardigrade Hypsibius dujardini, a new model for studying the evolution of development". Developmental Biology. 312 (2): 545–559. doi:10.1016/j.ydbio.2007.09.055. PMID 17996863.
- Fiona Macdonald (7 December 2015). "New Research Casts Doubt on The Claim That Tardigrades Get 1/6 of DNA From Other Species". ScienceAlert.
- Kinchin, IM (1987). "The moss fauna 1; Tardigrades". Journal of Biological Education. 21 (4): 288–90. doi:10.1080/00219266.1987.9654916.
- "13 ways Neil deGrasse Tyson's "Cosmos" sent the religious right off the deep end". 14 June 2014.
- "How the Quantum Realm could play into future Marvel films". 10 July 2018. Retrieved 29 July 2018.
- King, Darryn (6 July 2018). "The Science (and the Scientists) Behind 'Ant-Man'". The New York Times. Retrieved 29 July 2018.
- "Ant-Man and the Wasp needs a little help". 4 July 2018. Retrieved 29 July 2018.
Ant-Man and the Wasp is still intermittent fun, particularly for fans of tardigrades, the water-dwelling micro-fauna that had a brief cameo in the first Ant-Man, and get their well-deserved close-up in this one.
- "'Harbinger Down': New trailer for creature feature". Retrieved 3 October 2018.
- "'Harbinger Down' Review: A Bleak & Vanilla Creature Feature". bloody-disgusting.com. 31 July 2015. Retrieved 3 October 2018.
- Raftery, Brian (5 October 2016). "If You Only Read One Comic This Month, Make It 'Paper Girls'". Wired. Retrieved 8 August 2019.
- "Cosmo Sheldrake shares new single and tour dates". DIY. DIY. Retrieved 21 September 2019.
- "Cosmo Sheldrake - Tardigrade Song | Folk Radio". Folk Radio UK - Folk Music Magazine. 2 February 2015. Retrieved 21 September 2019.
- "The Scientific Truth About Ripper the 'Star Trek' Tardigrade Is a Huge Relief". Retrieved 5 September 2018.
- Salzberg, Steven. "New 'Star Trek' Series Makes Massive Science Blunder". Retrieved 5 September 2018.
- Placido, Dani Di. "'South Park' Review: Cartman Creates A Monster In 'Moss Piglets'". Retrieved 29 July 2018.
- "'South Park' season 21 episode 8 live stream: 'Moss Piglets'". 15 November 2017. Retrieved 29 July 2018.
- Nicole Yang (22 October 2018). "Kyrie Irving got a credit in the most recent episode of 'Family Guy'. Meet "Vernon The Water Bear."". Boston Globe Media Partners, LLC. Retrieved 22 October 2018.
- Webster, Andrew (1 November 2019). "Death Stranding is a long, bizarre journey that's both breathtaking and boring". The Verge. Retrieved 19 July 2020.
- Campbell, Colin (28 November 2019). "The A-Z of Death Stranding weirdness". Polygon (website). Retrieved 19 July 2020.
External links
| Wikispecies has information related to Tardigrada |
| Wikimedia Commons has media related to Tardigrada. |
- Tardigrade at the Encyclopædia Britannica
- Tardigrada Newsletter Archived 2015-03-20 at the Wayback Machine
- Tardigrades – Pictures and Movies Archived 2015-03-28 at the Wayback Machine
- The Edinburgh Tardigrade project Archived 2019-07-13 at the Wayback Machine
- The incredible water bear!
- Tardigrade Reference Center
- Tardigrades in space (TARDIS research project)
- Tardigrade data and analysis
- A short film about tardigrade research from NPR's Science Friday
- Tardigrada at the Tree of Life Web Project
- Swiss Center of Tardigrade Research – Ecology, Physiology and Evolutionary Biology of Tardigrades
- NASA Astronomy Picture of the Day: Tardigrade in Moss (6 March 2013)
- First Animal to Survive in Space, video (07:54), Vice Media
- Tardigrades are so tough, they can survive outer space (March 2015). BBC
- The International Society of Tardigrade Hunters
- Tardigrades discussed on Critter of the Week, Radio New Zealand
- How Marvel Studios created the water bears in Ant-Man and the Wasp!, video (03:04), Marvel Entertainment

