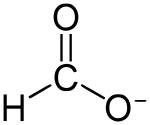
Formate
Formate (IUPAC name: methanoate) is the anion derived from formic acid. Its formula is represented in various equivalent ways: HCOO− or CHOO− or HCO2−. It is the product of deprotonation of formic acid. It is the simplest carboxylate anion. A formate (compound) is a salt or ester of formic acid.[1]
Biochemistry
Formate is reversibly oxidized by the enzyme formate dehydrogenase from Desulfovibrio gigas:[2]
- HCO2− → CO2 + H+ + 2 e−
Formate esters
Formate esters have the formula ROC(O)H (alternative way of writing formula RO2CH). Many form spontaneously when alcohols dissolve in formic acid.
The most important formate ester is methyl formate, which is produced as an intermediate en route to formic acid. Methanol and carbon monoxide react in the presence of a strong base, such as sodium methoxide:[1]
- CH3OH + CO → HCO2CH3
Hydrolysis of methyl formate gives formic acid and regenerates methanol:
- HCO2CH3 → HCO2H + CH3OH
Formic acid is used for many applications in industry.
Formate esters often are fragrant or have distinctive odors. Compared to the more common ethyl esters, formate esters are less commonly used commercially because they are less stable.[3] Ethyl formate is found in some confectionaries.[1]
Formate salts
Formate salts have the formula M(O2CH)(H2O)x. Such salts are prone to decarboxylation. For example, hydrated nickel formate decarboxylates at about 200 °C to give finely powdered nickel metal:
- Ni(O2CH)2(H2O)2 → Ni + 2 CO2 + 2 H2O + H2
Such fine powders are useful as hydrogenation catalysts.[1]
Examples
_formate_hydrate.jpg)
- ethyl formate, CH3CH2(HCOO)
- sodium formate, Na(HCOO)
- potassium formate, K(HCOO)
- caesium formate, Cs(HCOO); see Caesium: Petroleum exploration
- methyl formate, CH3(HCOO)
- methyl chloroformate, CH3OCOCl
- triethyl orthoformate
- trimethyl orthoformate, C4H10O3
- phenyl formate HCOOC6H5
- amyl formate
References
- Werner Reutemann and Heinz Kieczka "Formic Acid" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a12_013
- T. Reda, C. M. Plugge, N. J. Abram and J. Hirst, "Reversible interconversion of carbon dioxide and formate by an electroactive enzyme", PNAS 2008 105, 10654–10658. doi:10.1073/pnas.0801290105
- Johannes Panten and Horst Surburg "Flavors and Fragrances, 2. Aliphatic Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2015, Wiley-VCH, Weinheim.doi:10.1002/14356007.t11_t01