Paris green
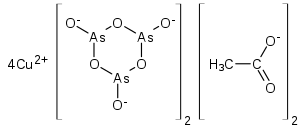
Paris green (copper(II) acetate triarsenite or copper(II) acetoarsenite) is an inorganic compound. As a green pigment it is also known as Schweinfurt green, emerald green or Vienna green. It is a highly toxic emerald-green crystalline powder[3] that has been used as a rodenticide and insecticide,[4] and also as a pigment, despite its toxicity. It is also used as a blue colorant for fireworks.[5] The color of Paris green is said to range from a pale blue green when very finely ground, to a deeper green when coarsely ground.
 | |
| Names | |
|---|---|
| Other names
C.I. pigment green 21, emerald green, Schweinfurt green, imperial green, Vienna green, Mitis green, Veronese green[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.125.242 |
PubChem CID |
|
| UNII | |
| UN number | 1585 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cu(C2H3O2)2·3Cu(AsO2)2 | |
| Molar mass | 1013.79444 g/mol |
| Appearance | Emerald green crystalline powder |
| Density | >1.1 g/cm3 (20 °C) |
| Melting point | > 345 °C (653 °F; 618 K) |
| Boiling point | decomposes |
| insoluble | |
| Solubility | soluble but unstable in acids insoluble in alcohol |
| Hazards | |
| Safety data sheet | CAMEO MSDS |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R23/25 R50/53 |
| S-phrases (outdated) | (S1/2) S20/21 S28 S45 S60 S61 |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
22 mg/kg |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
[1910.1018] TWA 0.010 mg/m3[2] |
REL (Recommended) |
Ca C 0.002 mg/m3 [15-minute][2] |
IDLH (Immediate danger) |
Ca [5 mg/m3 (as As)][2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Paris green | |
|---|---|
| Hex triplet | #50C878 |
| sRGBB (r, g, b) | (80, 200, 120) |
| CMYKH (c, m, y, k) | (60, 0, 40, 22) |
| HSV (h, s, v) | (140°, 60%, 78%) |
| Source | [Unsourced] |
| ISCC–NBS descriptor | Vivid yellowish green |
| B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) | |
Preparation
Paris green may be prepared by combining copper(II) acetate and arsenic trioxide.[6]
Invention
It was invented as 'emerald green' in 1814 by two chemists, Russ and Sattler, at the Wilhelm Dye and White Lead Company of Schweinfurt, Bavaria. They were attempting to produce an improved pigment over Scheele's green, particularly so that it was longer-lasting and less susceptible to darkening around sulfides.[lower-roman 1] When they published the recipe in 1822, its toxicity became obvious.[7] Despite this, like Scheele's green, it continued to be involved in poisoning accidents.[8]
Uses
Insecticide
In 1867, farmers in Illinois and Indiana found that Paris green was effective against the Colorado potato beetle, an aggressive agricultural pest. Despite concerns regarding the safety of using arsenic compounds on food crops, Paris green became the preferred method for controlling the beetle. By the 1880s, Paris green had become the first widespread use of a chemical insecticide in the world.[9] It was also used widely in the Americas to control the tobacco budworm, Heliothis virescens.[10]
Paris green was heavily sprayed by airplane in Italy, Sardinia, and Corsica during 1944 and in Italy in 1945 to control malaria.[11] It was once used to kill rats in Parisian sewers, which is how it acquired its common name.[12]
Pigment
Paris green, also called emerald green, was a popular pigment used in artists' paints by (among others) the English painter W. Turner, Impressionists such as Monet and Renoir, and Post-Impressionists such as Gauguin, Cézanne, and Van Gogh.[13]
Related pigments
Similar natural compounds are the minerals chalcophyllite Cu
18Al
2(AsO
4)
3(SO
4)
3(OH)
27·36H
2O, conichalcite CaCu(AsO
4)(OH), cornubite Cu
5(AsO
4)
2(OH)
4·H
2O, cornwallite Cu
5(AsO
4)
2(OH)
4·H
2O, and liroconite Cu
2Al(AsO
4)(OH)
4·4H
2O. These vivid minerals range from greenish blue to slightly yellowish green.
Scheele's green is a chemically simpler, less brilliant, and less permanent, synthetic copper-arsenic pigment used for a rather short time before Paris green was first prepared, which was approximately 1814. It was popular as a wallpaper pigment and would degrade, with moisture and molds, to arsine gas. Paris green was also used in wallpaper to some extent and may have also degraded similarly.[14] Both pigments were once used in printing ink formulations.
The ancient Romans used one of them, possibly conichalcite, as a green pigment. The Paris green paint used by the Impressionists is said to have been composed of relatively coarse particles. Later, the chemical was produced with increasingly small grinds and without carefully removing impurities; its permanence suffered. It is likely that it was ground more finely for use in watercolors and inks, too.
- Paris green pigment
 Mixing "Paris green" and road dust preparatory to dusting streams and breeding places of mosquitoes during World War II
Mixing "Paris green" and road dust preparatory to dusting streams and breeding places of mosquitoes during World War II Use as insecticide, poster issued by US Public Health Service
Use as insecticide, poster issued by US Public Health Service
See also
- List of colors
- List of inorganic pigments
References
- Sulfur was commonly produced from burning coal fires.
- "Health & Safety in the Arts -- Painting & Drawing Pigments". Environmental Management Division, City of Tucson AZ. Archived from the original on 2011-07-20. Retrieved 2011-02-07.
- NIOSH Pocket Guide to Chemical Hazards. "#0038". National Institute for Occupational Safety and Health (NIOSH).
- "Hazardous Substance Fact Sheet" (PDF). NJ Dept. of Health and Senior Services. Retrieved 2011-02-07.
- "Dangers in the Manufacture of Paris Green and Scheele's Green". Monthly Review of the U.s. Bureau of Labor Statistics. 5 (2): 78–83. 1917. JSTOR 41829377.
- "How to Use Copper in Pyro Star Compositions to Create Blue Fireworks Stars". Skylighter. Archived from the original on 2010-12-21. Retrieved 2011-02-07.
- "H.Wayne Richardson, "Copper Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a07_567
- Emsley, John (2005). The Elements of Murder: A History of Poison. OUP. p. 118. ISBN 9780192805997.
- Whorton, James C. (2010). The Arsenic Century: How Victorian Britain was Poisoned at Home, Work, and Play. OUP. p. 162. ISBN 9780191623431.
- Sorenson 1995
- Blanco, Carlos (2012). "Heliothis virescens and Bt cotton in the United States". GM Crops & Food: Biotechnology in Agriculture and the Food Chain. 3: 201–212.
- Justin M. Andrews, Sc. D. (1963). "Preventive Medicine in World War II, Chapter V. North Africa, Italy, and the Islands of the Mediterranean". Washington, D.C. USA: Office of the Surgeon General, Department of the Army. p. 281. Retrieved 2008-09-30.
- The Natural Paint Book, by Lynn Edwards, Julia Lawless, Table of contents
- Emerald green, Colourlex
- Atlas Obscura, "How a Library Handles a Rare and Deadly Book of Wallpaper Samples"
Further reading
- Fiedler, I. and Bayard, M. A., "Emerald Green and Scheele’s Green", in Artists' Pigments: A Handbook of Their History and Characteristics, Vol. 3: E.W. Fitzhugh (Ed.) Oxford University Press 1997, pp. 219–271
- Hughes, Michael F.; et al. (2011). "Arsenic Exposure and Toxicology: A Historical Perspective". Toxicological Sciences. 123 (2): 305–332. doi:10.1093/toxsci/kfr184. PMC 3179678. PMID 21750349.
- Sorensen, W. Conner (1995). Brethren of the Net, American Entomology, 1840-1880. University of Alabama Press. pp. 124–125.
- Spear, Robert J., The Great Gypsy Moth War, A History of the First Campaign in Massachusetts to Eradicate the Gypsy Moth, 1890-1901. University of Massachusetts Press, Amherst and Boston, 2005. ISBN 1-55849-479-0
External links
- Case Studies in Environmental Medicine - Arsenic Toxicity
- How Emerald green is made
- National Pollutant Inventory - Copper and compounds fact sheet
- Emerald green, Colourlex
Acetyl halides and salts of the acetate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||