Phthalocyanine Green G
Phthalocyanine green G, which has many commercial names, is a synthetic green pigment from the group of phthalocyanine dyes, a complex of copper(II) with chlorinated phthalocyanine. It is a soft green powder, which is insoluble in water.[1] It is a bright, high intensity colour used in oil and acrylic based artist's paints, and in other applications.
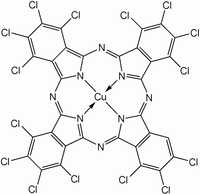 Chemical structure of one of the components of Phthalocyanine Green G | |
| Names | |
|---|---|
| Other names
Phthalo green, viridian hue, pigment green 7, copper phthalocyanine green, C.I. pigment green 7, non-flocculating green G, polychloro copper phthalocyanine, and C.I. 74260, copper hexadecachlorophthalocyanine | |
| Identifiers | |
| ECHA InfoCard | 100.014.114 |
| UNII | |
CompTox Dashboard (EPA) |
|
| Properties | |
| Variable | |
| Appearance | Green solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Phthalo green | |
|---|---|
 Phthalocyanine green pigment | |
| Hex triplet | #123524 |
| sRGBB (r, g, b) | (18, 53, 36) |
| CMYKH (c, m, y, k) | (82, 51, 80, 64) |
| HSV (h, s, v) | (151°, 66%, 21%) |
| Source | The Mother of All HTML Colo(u)r Charts |
| ISCC–NBS descriptor | Very dark yellowish green |
| B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) | |
Synthesis, properties, and production
Phthalocyanine green is derived from phthalocyanine blue by chlorination in the presence of aluminium trichloride. The stoichiometry for the complete chlorination is shown:[1]
- Cu(C32H16N8) + 16 Cl2 → Cu(C32N8Cl16) + 16 HCl
In practice, this pigment is a mixture of isomers and degrees of chlorination. The 15th and 16th chlorides are difficult to install. The chemical formula usually ranges from C32H3Cl13CuN8 to C32HCl15CuN8.
Due to the presence of strongly electronegative chlorine substituents, the absorption spectrum is shifted from that of the parent copper phthalocyanine. Phthalo green is highly stable and resistant to alkali, acids, solvents, heat, and ultraviolet radiation.
Uses
Due to its stability, phthalo green is used in inks, coatings, and many plastics. In application it is transparent. Being insoluble, it has no tendency to migrate in the material. It is a standard pigment used in printing ink and packaging industry. It is also allowed in all cosmetics except those used around the eyes. It is used in some tattoos.
Related compounds
Copper phthalocyanine green 36 is a variant where some of the chlorine atoms are replaced with bromine. Bromination is less efficient than chlorination. Consequently the degree of bromination is lower.[1][2]
See also
- The Joy of Painting - oil paint based on the pigment was frequently used on the show.
- List of colours
References
- Löbbert, Gerd (2000). "Phthalocyanines". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a20_213..
- Phthalo Green: How Can You Tell If It's Always the Same? 1/7/2003 , Dr. Richard M. Podhajny, Ph.D. , pffc-online.com