Acetyl fluoride
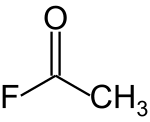
Acetyl fluoride is an acyl halide with the chemical formula CH3COF.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Acetyl fluoride | |
| Systematic IUPAC name
Ethanoyl fluoride | |
| Other names
Methylcarbonyl fluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.354 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H3FO | |
| Molar mass | 62.043 g·mol−1 |
| Density | 1.032 g/cm3 |
| Melting point | −84 °C (−119 °F; 189 K) |
| Boiling point | 21 °C (70 °F; 294 K)[1] |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Danger |
GHS hazard statements |
H314 |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Acetyl fluoride is synthesized using hydrogen fluoride and acetic anhydride. Acetic acid is produced as a byproduct.[3]
- HF + (CH
3CO)
2O → CH
3CO
2H + CH
3COF
gollark: Basically:- someone configures a channel as the "phone channel"- people can then dial that, or you can dial to other ones in it- if the other end accepts, a call is set up and messages are relayed between each channel until you hang up
gollark: You can use the very cool™ and useful™ feature of my Discord bot where you can open temporary "calls" to channels on other (participating) servers.
gollark: They are both Lua, essentially, but with different versions and APIs.
gollark: I don't trust wireless things much.
gollark: Also, 70kg, really?!
See also
References
- "Acetyl fluoride". Archived from the original on 2014-03-28. Retrieved 2012-03-07.
- "Acetyl Fluoride". NIST. Archived from the original on 21 February 2019. Retrieved 7 March 2012.
- Tanaka, Mutsuo; Fujiwara, Masahiro; Ando, Hisanori (1995). "Dual Reactivity of the Formyl Cation as an Electrophile and a Bransted Acid in Superacids". Journal of Organic Chemistry. 60 (12): 3846–3850. doi:10.1021/jo00117a041.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.