Manganese(II) acetate
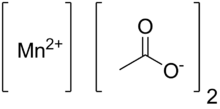
Manganese(II) acetate are chemical compounds with the formula Mn(CH3CO2)2.(H2O)n where n = 0, 2, 4.. It is used as a catalyst and as fertilizer.[3]
 | |
| Names | |
|---|---|
| IUPAC name
Manganese(II) acetate | |
| Other names
Manganese diacetate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.305 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Mn(CH3CO2)2 (anhydrous) Mn(CH3CO2)2·4H2O (tetrahydrate) | |
| Molar mass | 173.027 g/mol (anhydrous) 245.087 g/mol (tetrahydrate) |
| Appearance | white crystals (anhydrous) light pink monoclinic crystals (tetrahydrate) |
| Density | 1.74 g/cm3 (anhydrous) 1.59 g/cm3 (tetrahydrate) |
| Melting point | 210 °C (410 °F; 483 K) (anhydrous) 80 °C (tetrahydrate) |
| Solubility | soluble in water (about 700g/L at 20°C for tetrahydrate), methanol, acetic acid (anhydrous) soluble in water, ethanol (tetrahydrate) |
| +13,650·10−6 cm3/mol | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | > 130 °C (266 °F; 403 K) (tetrahydrate) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2940 mg/kg (rat, oral)[2] |
| Related compounds | |
Other anions |
Manganese(II) fluoride Manganese(II) chloride Manganese(II) bromide |
Other cations |
Zinc acetate Mercury(II) acetate Silver acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Reactions
Manganese(II) acetate can be formed by treating either manganese(II,III) oxide or manganese(II) carbonate with acetic acid:[4]
- MnCO3 + 2 CH3CO2H → Mn(CH3CO2)2 + CO2 + H2O
Structure
The anhydrous material and dihydrate Mn(CH3CO2)2.2H2O are coordination polymers. The dihydrate has been characterized by X-ray crystallography. Each Mn(II) center is surrounded by six oxygen centers provided by aquo ligands and acetates.
-acetate-dihydrate-unit-cell-3D-balls.png)
Subunit of the structure of the dihydrate of manganese(II) acetate.[5]
gollark: Vendor lock in, proprietary drivers, use of drivers to artificially segment the market.
gollark: Unless you're insulting those people in their capacity as developer of those languages, like the PHP person.
gollark: Insulting programming languages is cool and fun™, but you should avoid insulting people with them!
gollark: Oh, also, check out what I found recently: https://www.gnu.org/software/repo-criteria.html
gollark: I mean, unless you have specific glibc-uous requirements.
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–354, 4–68, ISBN 0-8493-0594-2
- "Manganese compounds (as Mn)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Thomas Scott; Mary Eagleson (1994), Concise encyclopedia chemistry, Walter de Gruyter, p. 620, ISBN 3-11-011451-8, retrieved 2009-07-20
- Arno H. Reidies (2002). "Manganese Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_123.CS1 maint: uses authors parameter (link)
- Chih-Yi Cheng, Sue-Lein Wang (1991). "Structure of manganese acetate dihydrate". Acta Crystallographica Section C. 47: 1734. doi:10.1107/S0108270191002202.CS1 maint: uses authors parameter (link)
Acetyl halides and salts of the acetate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
