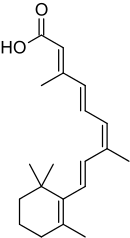
Alitretinoin
Alitretinoin, or 9-cis-retinoic acid, is a form of vitamin A. It is also used in medicine as an antineoplastic (anti-cancer) agent developed by Ligand Pharmaceuticals. It is a first generation retinoid. Ligand gained Food and Drug Administration (FDA) approval for alitretinoin in February 1999.
 | |
| Clinical data | |
|---|---|
| Trade names | Panretin (gel), Toctino (oral) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601012 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical, oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Highly bound, no exact figure available[1] |
| Metabolism | Hepatic (CYP3A4-mediated oxidation, also isomerised to tretinoin)[1] |
| Elimination half-life | 2-10 hours[1] |
| Excretion | Urine (64%), faeces (30%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.111.081 |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Medical uses
Kaposi’s sarcoma
In the United States, topical alitretinoin is indicated for the treatment of skin lesions in AIDS-related Kaposi's sarcoma. Alitretinoin is not indicated when systemic therapy against Kaposi's sarcoma is required.[2] It has received EMA (11 October 2000) and FDA (2 March 1999) approval for this indication.[3][4]
Chronic hand eczema
Alitretinoin has been granted prescription rights in the UK (08/09/2008) for in chronic hand eczema as used by mouth.[5] In May 2009 the National Institute for Health and Clinical Excellence (NICE) issued preliminary guidance[6] on the use of alitretinoin for the treatment of severe chronic hand eczema in adults. The recommendation stated that only patients with severe chronic hand eczema who are unresponsive to potent topical corticosteroids, oral immunosuppressants or phototherapy should receive the drug. Final NICE guidance is expected in August 2009.
Adverse effects
Systemic use
Very common (>10% frequency):
- Headache
- Hypertriglyceridemia
- High density lipoprotein decreased
- Hypercholesterolemia
Common (1-10% frequency):
- Anaemia
- Increased iron binding capacity
- Monocytes decreased
- Thrombocytes increased
- TSH decreased
- Free T4 decreased
- Flushing
- Conjunctivitis
- Dry eye
- Eye irritation
- Transaminase increased
- Dry skin
- Dry lips
- Cheilitis
- Eczema
- Dermatitis
- Erythema
- Hair loss
- Joint pain
- Muscle pains
- Blood creatine phosphokinase increased
Uncommon (0.1-1% frequency):
- Blurred vision
- Cataracts
- Nose bleeds
- Itchiness
- Rash
- Skin exfoliation
- Asteatotic eczema
- Exostosis
- Ankylosing spondylitis
Rare (<0.1% frequency):
- Benign intracranial hypertension
- Vasculitis
Unknown frequency:
- Anaphylactic reactions
- Hypersensitivity
- Depression
- Mood changes
- Suicidal ideation
- Decreased night vision
Topical use
Very common (>10% frequency):
- Rash (77%)
- Pain (34%)
- Itchiness (11%)
Common (1-10% frequency):
- Exfoliative dermatitis
- Oedema
- Skin changes
- Paraesthesia
Contraindications
Pregnancy is an absolute contraindication as with most other vitamin A products, it should also be avoided when it comes to systemic use in any women that is of childbearing potential and not taking precautions to prevent pregnancy.[1] Toctino (the oral capsule formulation of alitretinoin) contains soya oil and sorbitol. Patients who are allergic to peanut, soya or with rare hereditary fructose intolerance should not take this medicine.[1] It is also contraindicated in nursing mothers.[1] The oral formulation of alitretinoin is contraindicated in patients with:[1]
- Hepatic insufficiency
- Severe chronic kidney disease
- Uncontrolled hypercholesterolemia
- Uncontrolled hypertriglyceridemia
- Uncontrolled hypothyroidism
- Hypervitaminosis A
- Hypersensitivity to any excipients in alitretinoin
Interactions
It is a CYP3A4 substrate and hence any inhibitor or inducer of this enzyme may alter plasma levels of alitretinoin.[1] It should not be given to patients with excess vitamin A in their system as there is a potential for its actions on the retinoid X receptor to be exacerbated.[1] It may also interact with tetracyclines to cause benign intracranial hypertension.[1]
Overdose
Alitretinoin is a form of vitamin A. Alitretinoin has been administered in oncological clinical studies at dosages of more than 10-times of the therapeutic dosage given for chronic hand eczema. The adverse effects observed were consistent with retinoid toxicity, and included severe headache, diarrhoea, facial flushing and hypertriglyceridemia. These effects were reversible.[1]
Mechanism of action
Alitretinoin is believed to be the endogenous ligand (a substance that naturally occurs in the body that activates this receptor) for retinoid X receptor, but it also activates the retinoic acid receptor.[1][8][9]
References
- "Toctino 10mg and 30mg soft capsules - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Stiefel. 30 August 2013. Retrieved 1 February 2014.
- "Panretin (Alitretinoin) Drug Information". RxList. November 21, 2000. Archived from the original on 18 December 2008. Retrieved 2009-01-14.
- "Panretin : EPAR - Product Information" (PDF). European Medicines Agency. Eisai Ltd. 14 September 2012.
- "PANRETIN (alitretinoin) gel [Eisai Inc.]". DailyMed. Eisai Inc. March 2012. Retrieved 1 February 2014.
- Ruzicka T, Larsen FG, Galewicz D, Horváth A, Coenraads PJ, Thestrup-Pedersen K, et al. (December 2004). "Oral alitretinoin (9-cis-retinoic acid) therapy for chronic hand dermatitis in patients refractory to standard therapy: results of a randomized, double-blind, placebo-controlled, multicenter trial". Archives of Dermatology. 140 (12): 1453–9. doi:10.1001/archderm.140.12.1453. PMID 15611422.
- "NICE guidance documentation".
- "Panretin, (alitretinoin topical), dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 1 February 2014.
- Rowe A (February 1997). "Retinoid X receptors". The International Journal of Biochemistry & Cell Biology. 29 (2): 275–8. doi:10.1016/S1357-2725(96)00101-X. PMID 9147128.
- Dawson MI, Xia Z (January 2012). "The retinoid X receptors and their ligands". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1821 (1): 21–56. doi:10.1016/j.bbalip.2011.09.014. PMC 4097889. PMID 22020178.
External links
- Prescribing Information (revised February 2007). Retrieved on 01-30-08.