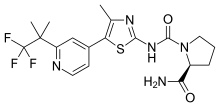
Alpelisib
Alpelisib, sold under the brand name Piqray, is a medication sold by Novartis and used to treat certain types of breast cancer.[1] It is used together with fulvestrant.[1] It is taken by mouth.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Piqray |
| Other names | BYL719 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619036 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.233.704 |
| Chemical and physical data | |
| Formula | C19H22F3N5O2S |
| Molar mass | 441.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Common side effects include high blood sugar, kidney problems, diarrhea, rash, low blood cells, liver problems, pancreatitis, vomiting, and hair loss.[1] It is an alpha-specific PI3K inhibitor.[1][2] It was approved for medical use in the United States in May 2019.[1]
Medical uses
In the European Union alpelisib will be indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)‑positive, human epidermal growth factor receptor 2 (HER2)‑negative, locally advanced or metastatic breast cancer with a PIK3CA mutation after disease progression following endocrine therapy as monotherapy.[3]
History
In May 2019, alpelisib was approved in the United States for use in combination with the endocrine therapy fulvestrant, to treat postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer following progression on or after an endocrine-based regimen.[1]
The U.S. Food and Drug Administration (FDA) also approved the companion diagnostic test, therascreen PIK3CA RGQ PCR Kit, to detect the PIK3CA mutation in a tissue and/or a liquid biopsy.[1]
The efficacy of alpelisib was studied in the SOLAR-1 trial (NCT02437318), a randomized trial of 572 postmenopausal women and men with HR-positive, HER2-negative, advanced or metastatic breast cancer whose cancer had progressed while on or after receiving an aromatase inhibitor.[1][4]
The FDA granted the application for alpelisib priority review designation and granted approval of Piqray to Novartis. The FDA granted approval of the therascreen PIK3CA RGQ PCR Kit to Qiagen Manchester, Ltd.[1]
On 28 May 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product alpelisib (Piqray), intended for the treatment of locally advanced or metastatic breast cancer with a PIK3CA mutation.[3] The applicant for this medicinal product is Novartis Europharm Limited.[3]
References
- "FDA approves first PI3K inhibitor for breast cancer". U.S. Food and Drug Administration (FDA) (Press release). 24 May 2019. Archived from the original on 25 November 2019. Retrieved 29 May 2019.

- André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. (May 2019). "PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer". The New England Journal of Medicine. 380 (20): 1929–1940. doi:10.1056/NEJMoa1813904. PMID 31091374.
- "Piqray: Pending EC decision". European Medicines Agency (EMA). 28 May 2020. Retrieved 23 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Drug Trials Snapshots: Piqray". U.S. Food and Drug Administration (FDA). 14 June 2019. Archived from the original on 25 November 2019. Retrieved 24 November 2019.

External links
- "Alpelisib". Drug Information Portal. U.S. National Library of Medicine.
- "Drug Approval Package: Piqray". U.S. Food and Drug Administration (FDA).