Carmofur
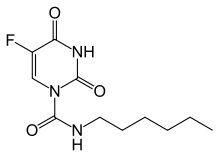
Carmofur (INN) or HCFU (1-hexylcarbamoyl-5-fluorouracil) is a pyrimidine analogue used as an antineoplastic agent. It is a derivative of fluorouracil, being a lypophilic-masked analog of 5-FU that can be administered orally.[1]
 | |
| Clinical data | |
|---|---|
| Other names | 1-hexylcarbamoyl-5-fluorouracil, HCFU, N-hexylcarbamoyl-5-fluorouracil, Yamaful, NCGC00095165-01, Hexylcarbamoyl fluorouracil, 61422-45-5, UNII-HA82M3RAB2, CCRIS 2759, C11H16FN3O3, Uracil, 5-fluoro-1-hexylcarbamoyl-, BRN 0888898, HA82M3RAB2, 1(2H)-Pyrimidinecarboxamide, 5-fluoro-N-hexyl-3,4, |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.216.315 |
| Chemical and physical data | |
| Formula | C11H16FN3O3 |
| Molar mass | 257.265 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Biology
Carmofur prodrug is ingested and taken up in the intestine, overcoming the problem of 5-FU degradation by dihydropyrimidine dehydrogenase. Once inside a cell, carmofur prodrug is converted into 5-FU.
Mechanism of action
The mechanism of action of carmofur prodrug is traditionally thought to be the generation of 5–FU.[2] However, carmofur is a highly potent acid ceramidase (AC) inhibitor.[2] Ceramide influences cancer cell survival, growth and death.[2] Inhibition of AC activity sensitizes tumor cells to the effects of antineoplastic agents and radiation.[2] Carmofur, much more effective than temozolomide, has been reported as the small-molecule drug capable of killing adult and pediatric glioblastomas.[3][4]
Medicinal uses
Product marketing for carmofur started in 1981. Carmofur has also been used as adjuvant chemotherapy for curatively resected colorectal cancer patients in China, Japan, and Finland for many years.[5] Trials and meta-analyses have confirmed that the drug is effective on patients with this cancer type, extending their survival.[6]
Carmofur has been shown to inhibit the SARS-CoV-2 main protease, and is therefore a promising lead compound to develop new antiviral treatment for COVID-19.[7]
Adverse effects
As fluorouracil, carmofur has been known to induce leukoencephalopathy, characterized by progressive damage to white matter in the brain with stroke-like symptoms.[8][9][10]
A clinical trial for small hepatocellular carcinoma was stopped prematurely because 56% of the treated patients had unacceptable side effects. Moreover, the treatment had no survival advantage for stage 1 and 2 cancer patients.[11] This may be a reason why carmofur was never pursued for FDA-approval in the US.[1]
Chemical synthesis
Ozaki et al. has reported carmofur synthesized by treating 5-FU with phosgene and hexylamine.[12] Xiong et al. reported an alternative approach for carmofur synthesis. Chemical preparations and structures can be found here.[1]
References
- Shelton J, Lu X, Hollenbaugh JA, Cho JH, Amblard F, Schinazi RF (Dec 2016). "Metabolism, Biochemical Actions, and Chemical Synthesis of Anticancer Nucleosides, Nucleotides, and Base Analogs". Chem Rev. 116 (23): 14379–14455. doi:10.1021/acs.chemrev.6b00209. PMID 27960273.
- Realini, Natalia; Solorzano, Carlos; Pagliuca, Chiara; Pizzirani, Daniela; Armirotti, Andrea; Luciani, Rosaria; Paola Costi, Maria; Bandiera, Tiziano; Piomelli, Daniele (Jan 2013). "Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity". Scientific Reports. 3 (1035): 1035. doi:10.1038/srep01035. PMC 3539145. PMID 23301156.
- Doan, Ninh B.; Nguyen, Ha S.; Montoure, Andrew; Al-Gizawiy, Mona M.; Mueller, Wade M.; Kurpad, Shekar; Rand, Scott D.; Connelly, Jennifer M.; Chitambar, Christopher R. (2017-03-01). "Acid ceramidase is a novel drug target for pediatric brain tumors". Oncotarget. 8 (15): 24753–24761. doi:10.18632/oncotarget.15800. ISSN 1949-2553. PMC 5421885. PMID 28445970.
- Doan, Ninh B.; Alhajala, Hisham; Al-Gizawiy, Mona M.; Mueller, Wade M.; Rand, Scott D.; Connelly, Jennifer M.; Cochran, Elizabeth J.; Chitambar, Christopher R.; Clark, Paul (2017-11-23). "Acid ceramidase and its inhibitors: a de novo drug target and a new class of drugs for killing glioblastoma cancer stem cells with high efficiency". Oncotarget. 8 (68). doi:10.18632/oncotarget.22637. ISSN 1949-2553. PMC 5762539. PMID 29348854.
- Sakamoto J, Oba K, Matsui T, Kobayashi M (Oct 2006). "Efficacy of oral anticancer agents for colorectal cancer". Dis. Colon Rectum. 49 (10 Suppl): S82–91. doi:10.1007/s10350-006-0601-7. PMID 17106820.
- Sakamoto, J; Hamada, C; Rahman, M; Kodaira, S; Ito, K; Nakazato, H; Ohashi, Y; Yasutomi, M (2005). "An Individual Patient Data Meta-analysis of Adjuvant Therapy with Carmofur in Patients with Curatively Resected Colon Cancer". Japanese Journal of Clinical Oncology. 35 (9): 536–544. doi:10.1093/jjco/hyi147. PMID 16155120.
- "Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur". Nature Structural and Molecular Biology. Dec 2020. doi:10.1038/s41594-020-0440-6.
- Yamada T, Okamura S, Okazaki T, et al. (June 1989). "Leukoencephalopathy following treatment with carmofur: a case report and review of the Japanese literature". Asia Oceania J Obstet Gynaecol. 15 (2): 161–8. doi:10.1111/j.1447-0756.1989.tb00171.x. PMID 2667512.
- Mizutani T (February 2008). "[Leukoencephalopathy caused by antineoplastic drugs]". Brain Nerve (in Japanese). 60 (2): 137–41. PMID 18306661.
- Baehring JM, Fulbright RK (May 2008). "Delayed leukoencephalopathy with stroke-like presentation in chemotherapy recipients". J Neurol Neurosurg Psychiatry. 79 (5): 535–9. doi:10.1136/jnnp.2007.123737. PMID 17682013.
- Yamamoto M, Arii S, Sugahara K, Tobe T (Mar 1996). "Adjuvant oral chemotherapy to prevent recurrence after curative resection for hepatocellular carcinoma". Br J Surg. 83 (3): 336–40. doi:10.1002/bjs.1800830313. PMID 8665186.
- Ozaki S, Nagase T, Ahmad S, Tamai H, Hoshi A, Iigo M (1987). "Synthesis and antitumor activity of 1- or 3-(alpha-hetero substituted)alkyl-5-fluorouracil derivatives". Nucleic Acids Symp Ser. 18 (18): 1–4. PMID 3697106.