Electrochromic devices
An electrochromic device (ECD) controls optical properties such as optical transmission, absorption, reflectance and/or emittance in a continual but reversible manner on application of voltage (electrochromism). This property enables an ECD to be used for applications like smart glass, electrochromic mirrors, and electrochromic display devices.
History
The history of coloration goes back to 1704 when Diesbach discovered Prussian blue (hexacyanoferrate), which changes the color from transparent to blue under oxidation of iron. In the 1930s, Kobosew and Nekrassow first noted electrochemical coloration in bulk tungsten oxide. While working at Balzers in Lichtenstein, T. Kraus provided a detailed description of electrochemical coloration in thin film of tungsten trioxide (WO3) on 30 July 1953. In 1969, S. K. Deb demonstrated electrochromic coloration in WO3 thin films.[1] Deb observed electrochromic color by applying electric field of the order of 104 Vcm−1 across WO3 thin film. In fact, the real birth of the EC technology is usually attributed to S. K. Deb’s seminal paper of 1973, wherein he described the coloration mechanism in WO3.[2] The electrochromism occurs due to the electrochemical redox reactions that take place in electrochromic materials. Various types of materials and structures can be used to construct electrochromic devices, depending on the specific applications.
Device structure
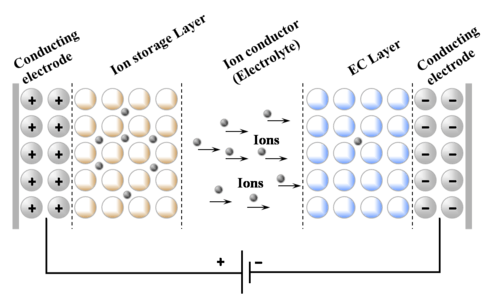
Electrochromic (sometimes called electrochromatic) devices are one kind of electrochromic cells.[3] The basic structure of ECD consists of two EC layers separated by an electrolytic layer. The ECD works on an external voltage, for which the conducting electrodes are used on the either side of both EC layers. Electrochromic devices can be categorized in two types depending upon the kind of electrolyte used viz. Laminated ECD are the one in which liquid gel is used while in solid electrolyte EC devices solid inorganic or organic material is used. The basic structure of electrochromic device embodies five superimposed layers on one substrate or positioned between two substrates in a laminated configuration. In this structure there are three principally different kinds of layered materials in the ECD: The EC layer and ion-storage layer conduct ions and electrons and belong to the class of mixed conductors. The electrolyte is a pure ion conductor and separates the two EC layers. The transparent conductors are pure electron conductors. Optical absorption occurs when electrons move into the EC layers from the transparent conductors along with charge balancing ions entering from the electrolyte.
Solid-state devices
In solid-state electrochromic devices, a solid inorganic or organic material is used as the electrolyte. Ta2O5 and ZrO2 are the most extensively studied inorganic solid electrolytes.
Laminated devices
Laminated electrochromic devices contain a liquid gel which is used as the electrolyte.
Mode of operation
_Operation.png)
Typically, ECD are of two types depending on the modes of device operation, namely the transmission mode and reflectance mode. In the transmission mode, the conducting electrodes are transparent and control the light intensity passing through them; this mode is used in smart-window applications. In the reflectance mode, one of the transparent conducting electrodes (TCE) is replaced with a reflective surface like aluminum, gold or silver, which controls the reflective light intensity; this mode is useful in rear-view mirrors of cars and EC display devices.
Applications
Smart windows

Electrochromic windows, also known as smart windows, are a new technological arrangement for achieving energy efficiency in buildings, with variable transmittance of light and solar energy. These ‘‘smart windows’’ can automatically control the amount of light and solar energy passing through the windows which subsequently improves indoor comfort; for example, electrochromic glass provides better glare resistance than fritted glass in most direct sunlight applications.[4] The efficiency of these windows will vary depending on their placement, size, and local climate conditions since these factors influence the amount of sunlight that comes in contact with these windows.[5] Electrochromic windows generally achieve their control over light and heat through their layered design. These layers within the window allow for tinting of the glass in response to increases in incoming sunlight as well as protection against UV radiation. An example of this layered design is the electrochromic glass developed by Gesimat, where multiple layers of material (i.e. tungsten oxide, polyvinyl butyral, and Prussian Blue) are sandwiched by two dual layers of glass and fluorine-doped tin oxide-coated glass.[6] Together, the tungsten oxide and Prussian Blue layers form complementary electrochromic layers; essentially, this means they form the positive and negative ends of a battery using the incoming solar energy.[7] The polyvinyl butyral (PVB) forms the central layer in this configuration, and it serves as a polymer electrolyte (this allows for the flow of ions which, in turn, generates a current).
Mirrors
Electrochromic reflecting surfaces are employed as self darkening mirrors that regulate reflections of flashing light from following vehicles at night so that a driver can see them without discomfort.
Other displays
Electrochromic displays can operate in either reflecting or transmitting mode. They are advantageous for their low cost and low power consumption.
There are many applications where they can be used, for example, in goggles and motorcycle helmet visors, which can be dynamically tinted depending on the time of day, and in paper, to create an image upon touching it with a stylus.
Gallery
 An electrochromic slide in bleached state
An electrochromic slide in bleached state An electrochromic slide in colored state
An electrochromic slide in colored state- Electrochromic glass installed in buildings
 An auto-dimming car mirror
An auto-dimming car mirror
References
- S. K. Deb, Appl. Opt., 8(S1) (1969) 192
- S. K. Deb, Phil. Mag., 27 (1973) 801
- Xu, Jian Wei; Chua, Ming Hui; Shah, Kowk Wei (January 2019). Electrochromic Smart Materials: Fabrication and Applications. Royal Society of Cambridge. doi:10.1039/9781788016667. ISBN 978-1-78801-143-3. Retrieved 22 August 2019.
- Malekafzali Ardakan, Ahoo; Sok, Eloïse; Niemasz, Jeff (2017-09-01). "Electrochromic glass vs. fritted glass: an analysis of glare control performance". Energy Procedia. 122: 343–348. doi:10.1016/j.egypro.2017.07.334. ISSN 1876-6102.
- Aldawoud, Abdelsalam (2013-04-01). "Conventional fixed shading devices in comparison to an electrochromic glazing system in hot, dry climate". Energy and Buildings. 59: 104–110. doi:10.1016/j.enbuild.2012.12.031. ISSN 0378-7788.
- Kraft, Alexander; Rottmann, Matthias; Heckner, Karl-Heinz (2006-03-06). "Large-area electrochromic glazing with ion-conducting PVB interlayer and two complementary electrodeposited electrochromic layers". Solar Energy Materials and Solar Cells. 90 (4): 469–476. doi:10.1016/j.solmat.2005.01.019. ISSN 0927-0248.
- Kraft, Alexander; Rottmann, Matthias (2009-12-01). "Properties, performance and current status of the laminated electrochromic glass of Gesimat". Solar Energy Materials and Solar Cells. 93 (12): 2088–2092. doi:10.1016/j.solmat.2009.05.010. ISSN 0927-0248.