Molybdenum trioxide
Molybdenum trioxide is chemical compound with the formula MoO3. This compound is produced on the largest scale of any molybdenum compound. It is an intermediate in the production of molybdenum metal. It is also an important industrial catalyst.[7] Molybdenum trioxide occurs as the rare mineral molybdite.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Molybdenum trioxide | |||
| Other names | |||
| Identifiers | |||
| ECHA InfoCard | 100.013.823 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| Properties | |||
| MoO3 | |||
| Molar mass | 143.95 g·mol−1 | ||
| Appearance | yellow or light blue solid | ||
| Odor | odorless | ||
| Density | 4.70 g/cm3[1] | ||
| Melting point | 802 °C (1,476 °F; 1,075 K)[1] | ||
| Boiling point | 1,155 °C (2,111 °F; 1,428 K)(sublimes)[1] | ||
| 1.066 g/L (18 °C) 4.90 g/L (28 °C) 20.55 g/L (70 °C) | |||
| Band gap | >3 eV (direct)[2] | ||
| +3.0·10−6 cm3/mol[3] | |||
| Structure[4] | |||
| Orthorhombic, oP16 | |||
| Pnma, No. 62 | |||
a = 1.402 nm, b = 0.37028 nm, c = 0.39663 nm | |||
Formula units (Z) |
4 | ||
| see text | |||
| Thermochemistry[5] | |||
Heat capacity (C) |
75.0 J K−1 mol−1 | ||
Std molar entropy (S |
77.7 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
−745.1 kJ/mol | ||
Gibbs free energy (ΔfG˚) |
-668.0 kJ/mol | ||
| Hazards | |||
| Safety data sheet | See: data page | ||
EU classification (DSD) (outdated) |
Carc. Cat. 3 Harmful (Xn) Irritant (Xi) | ||
| R-phrases (outdated) | R36/37, R40 | ||
| S-phrases (outdated) | (S2), S22, S36/37 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
125 mg.kg (rat, oral) 2689 mg/kg (rat, oral)[6] | ||
LDLo (lowest published) |
120 mg Mo/kg (rat, oral) 120 mg Mo/kg (guinea pig, oral)[6] | ||
LC50 (median concentration) |
>5840 mg/m3 (rat, 4 hr)[6] | ||
| Related compounds | |||
Other cations |
Chromium trioxide Tungsten trioxide | ||
Related molybdenum oxides |
Molybdenum dioxide "Molybdenum blue" | ||
Related compounds |
Molybdic acid Sodium molybdate | ||
| Supplementary data page | |||
Structure and properties |
Refractive index (n), Dielectric constant (εr), etc. | ||
Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
Spectral data |
UV, IR, NMR, MS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Structure
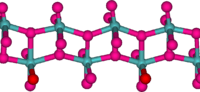
In the gas phase, three oxygen atoms are double bonded to the central molybdenum atom. In the solid state, anhydrous MoO3 is composed of layers of distorted MoO6 octahedra in an orthorhombic crystal. The octahedra share edges and form chains which are cross-linked by oxygen atoms to form layers. The octahedra have one short molybdenum-oxygen bond to a non-bridging oxygen.[8][9] Also known is a metastable (β) form of MoO3 with a WO3-like structure.[10][2]
Preparation and principal reactions
MoO3 is produced industrially by roasting molybdenum disulfide, the chief ore of molybdenum:[7]
- 2 MoS2 + 7 O2 → 2 MoO3 + 4 SO2
The laboratory synthesis of the dihydrate entails acidification of aqueous solutions of sodium molybdate with perchloric acid:[11]
- Na2MoO4 + H2O + 2 HClO4 → MoO3(H2O)2 + 2 NaClO4
The dihydrate loses water readily to give the monohydrate. Both are bright yellow in color.
Molybdenum trioxide dissolves slightly in water to give "molybdic acid". In base, it dissolves to afford the molybdate anion.
Uses
Molybdenum trioxide is used to manufacture molybdenum metal, which serves as an additive to steel and corrosion-resistant alloys. The relevant conversion entails treatment of MoO3 with hydrogen at elevated temperatures:
- MoO3 + 3 H2 → Mo + 3 H2O
It is also a component of the co-catalyst used in the industrial production of acrylonitrile by the oxidation of propene and ammonia.
Because of its layered structure and the ease of the Mo(VI)/Mo(V) coupling, MoO3 is of interest in electrochemical devices and displays.[12] Molybdenum trioxide has also been suggested as a potential anti-microbial agent, e.g., in polymers. In contact with water, it forms H+ ions that can kill bacteria effectively.[13]

References
- Haynes, p. 4.77
- Balendhran, Sivacarendran; Walia, Sumeet; Nili, Hussein; Ou, Jian Zhen; Zhuiykov, Serge; Kaner, Richard B.; Sriram, Sharath; Bhaskaran, Madhu; Kalantar-zadeh, Kourosh (2013-08-26). "Two-Dimensional Molybdenum Trioxide and Dichalcogenides". Advanced Functional Materials. 23 (32): 3952–3970. doi:10.1002/adfm.201300125.
- Haynes, p. 4.134
- Åsbrink, S.; Kihlborg, L. and Malinowski, M. (1988). "High-pressure single-crystal X-ray diffraction studies of MoO3. I. Lattice parameters up to 7.4 GPa". J. Appl. Cryst. 21: 960–962. doi:10.1107/S0021889888008271.CS1 maint: multiple names: authors list (link)
- Haynes, p. 5.15
- "Molybdenum (soluble compounds, as Mo)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Roger F. Sebenik et al. (2005). "Molybdenum and Molybdenum Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_655. ISBN 978-3527306732.CS1 maint: uses authors parameter (link)
- "Molybdite Mineral Data". Webmineral.
- Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- McCarron, E. M. (1986). "β-MoO3: A Metastable Analogue of WO3". J. Chem. Soc., Chem. Commun.: 336–338. doi:10.1039/C39860000336.
- Heynes, J. B. B.; Cruywagen, J. J. (1986). "Yellow Molybdenum(VI) Oxide Dihydrate". Inorganic Syntheses. 24: 191–2. doi:10.1002/9780470132555.ch56. ISBN 9780470132555.
- Ferreira, F. F.; Souza Cruz, T. G.; Fantini, M. C. A.; Tabacniks, M. H.; de Castro, S. C.; Morais, J.; de Siervo, A.; Landers, R.; Gorenstein, A. (2000). "Lithium insertion and electrochromism in polycrystalline molybdenum oxide films". Solid State Ionics. 136–137 (1–2): 357–363. doi:10.1016/S0167-2738(00)00483-5.
- Zollfrank, Cordt; Gutbrod, Kai; Wechsler, Peter; Guggenbichler, Josef Peter (2012). "Antimicrobial activity of transition metal acid MoO3 prevents microbial growth on material surfaces". Materials Science and Engineering: C. 32 (1): 47–54. doi:10.1016/j.msec.2011.09.010. PMID 23177771.
Cited sources
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. ISBN 978-1439855119.
External links
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- U.S. Department of Health and Human Services National Toxicology Program
- International Molybdenum Association
- Los Alamos National Laboratory – Molybdenum
-oxid_Kristallstruktur.png)

