Sodium ferrocyanide
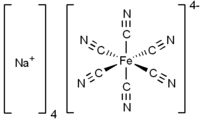
Sodium ferrocyanide is the sodium salt of the coordination compound of formula [Fe(CN)6]4−. In its hydrous form, Na4Fe(CN)6 • 10H2O (sodium ferrocyanide decahydrate), it is sometimes known as yellow prussiate of soda. It is a yellow crystalline solid that is soluble in water and insoluble in alcohol. The yellow color is the color of ferrocyanide anion. Despite the presence of the cyanide ligands, sodium ferrocyanide has low toxicity (acceptable daily intake 0–0.025 mg/kg body weight[2]). The ferrocyanides are less toxic than many salts of cyanide, because they tend not to release free cyanide.[3] However, like all ferrocyanide salt solutions, addition of an acid can result in the production of hydrogen cyanide gas, which is toxic.
 | |
| Names | |
|---|---|
| IUPAC name
Tetrasodium [hexacyanoferrate(II)] | |
| Other names
white prussiate of soda (YPS), Tetrasodium hexacyanoferrate, Gelbnatron, Ferrocyannatrium, sodium hexacyanoferrate(II), Yellow blood salt | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.033.696 |
| EC Number |
|
| E number | E535 (acidity regulators, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Na4[Fe(CN)6] | |
| Molar mass | 303.91 g/mol |
| Appearance | pale yellow crystals |
| Odor | odorless |
| Density | 1.458 g/cm3 |
| Melting point | 435 °C (815 °F; 708 K) (anhydrous) 81.5 °C (178.7 °F; 354.6 K) (decahydrate) (decomposes) |
| 10.2 g/100 mL (10 °C) 17.6 g/100 mL (20 °C) 39.7 g/100 mL (96.6 °C) | |
Refractive index (nD) |
1.530 |
| Structure | |
| monoclinic | |
| Hazards | |
| S-phrases (outdated) | S22 S24 S25 |
| Related compounds | |
Other anions |
Sodium ferricyanide (Red prussiate of soda) |
Other cations |
Potassium ferrocyanide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
When combined with iron, it converts to a deep blue pigment called Prussian blue.[4] It is used as a stabilizer for the coating on welding rods. In the petroleum industry, it is used for removal of mercaptans.
In the EU, ferrocyanides (E 535–538) were, as of 2018, solely authorised in two food categories as salt substitutes. Kidneys are the organ for ferrocyanide toxicity.[5]
Production
Sodium ferrocyanide is produced industrially from hydrogen cyanide, ferrous chloride, and calcium hydroxide, the combination of which affords Ca2[Fe(CN)6] • 11H2O. A solution of this salt is then treated with sodium salts to precipitate the mixed calcium-sodium salt CaNa2[Fe(CN)6], which in turn is treated with sodium carbonate to give the tetrasodium salt.[6]
References
- Sodium ferrocyanide MSDS Archived 2010-05-17 at the Wayback Machine
- "Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents". World Health Organization, Geneva. 1974. Retrieved 18 May 2009.
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- "Prussian blue". Encyclopædia Britannica. Retrieved 18 May 2009.
- Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert‐Remy, Gunter Georg Kuhnle, Claude Lambré, Jean‐Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens‐Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes. (2018). "Re‐evaluation of sodium ferrocyanide (E 535), potassium ferrocyanide (E 536) and calcium ferrocyanide (E 538) as food additives". EFSA Journal. 16 (7): 5374. doi:10.2903/j.efsa.2018.5374.CS1 maint: multiple names: authors list (link)
- Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M.; Kellens, R.; Reddy, J.; Steier, N.; Hasenpusch, W. (2011). "Cyano Compounds, Inorganic". Ullmann's Encyclopedia in Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_159.pub3.