Chromium(III) oxide
Chromium(III) oxide (or chromia) is an inorganic compound with the formula Cr
2O
3. It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.
 | |
 | |
| Names | |
|---|---|
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.783 |
| EC Number |
|
| 11116 | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cr2O3 | |
| Molar mass | 151.9904 g/mol |
| Appearance | light to dark green, fine crystals |
| Density | 5.22 g/cm3 |
| Melting point | 2,435 °C (4,415 °F; 2,708 K) |
| Boiling point | 4,000 °C (7,230 °F; 4,270 K) |
| insoluble | |
| Solubility in alcohol | insoluble in alcohol, acetone, acids |
| +1960.0×10−6 cm3/mol | |
Refractive index (nD) |
2.551 |
| Structure | |
| hexagonal | |
| R3c, No. 167[1] | |
| Thermochemistry | |
Std molar entropy (S |
81 J·mol−1·K−1 |
Std enthalpy of formation (ΔfH⦵298) |
−1128 kJ·mol−1 |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H302, H317, H319, H360 |
| P201, P202, P261, P264, P270, P272, P280, P281, P301+312, P302+352, P305+351+338, P308+313, P321, P330, P333+313, P337+313, P363, P405, P501 | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 1 mg/m3[2] |
REL (Recommended) |
TWA 0.5 mg/m3[2] |
IDLH (Immediate danger) |
250 mg/m3[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
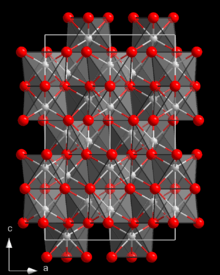
Structure and properties
Cr
2O
3 has the corundum structure, consisting of a hexagonal close packed array of oxide anions with 2⁄3 of the octahedral holes occupied by chromium. Similar to corundum, Cr
2O
3 is a hard, brittle material (Mohs hardness 8 to 8.5).[3]
It is antiferromagnetic up to 307 K, the Néel temperature.[4][5] It is not readily attacked by acids.
Occurrence

Cr
2O
3 occurs naturally as the mineral eskolaite, which is found in chromium-rich tremolite skarns, metaquartzites, and chlorite veins. Eskolaite is also a rare component of chondrite meteorites. The mineral is named after Finnish geologist Pentti Eskola.[3]
Production
The Parisians Pannetier and Binet first prepared the transparent hydrated form of Cr
2O
3 in 1838 via a secret process, sold as a pigment.[6] It is derived from the mineral chromite, (Fe,Mg)Cr
2O
4. The conversion of chromite to chromia proceeds via Na
2Cr
2O
7, which is reduced with sulfur at high temperatures:[7]
- Na
2Cr
2O
7 + S → Na
2SO
4 + Cr
2O
3
The oxide is also formed by the decomposition of chromium salts such as chromium nitrate, or by the exothermic decomposition of ammonium dichromate.
- (NH
4)
2Cr
2O
7 → Cr
2O
3 + N
2 + 4 H
2O
The reaction has a low ignition temperature of less than 200 °C and is frequently used in “volcano” demonstrations.[8]
Applications
Because of its considerable stability, chromia is a commonly used pigment. It was originally called viridian. It is used in paints, inks, and glasses. It is the colorant in "chrome green" and "institutional green." Chromium(III) oxide is a precursor to the magnetic pigment chromium dioxide, by the following reaction:[7]
- Cr
2O
3 + 3 CrO
3 → 5 CrO
2 + O
2
Along with many other oxides, it is used as a compound when polishing (also called stropping) the edges of knives, razors, surfaces of optical devices etc. on a piece of leather, balsa, cloth or other material. It is available in powder or wax form, and in this context it is known as "green compound".
Reactions
Chromium(III) oxide is amphoteric. Although insoluble in water, it reacts with acid to produce salts of hydrated chromium ions such as [Cr(H
2O)
6]3+
.[9] It is also attacked by concentrated alkali to yield salts of [Cr(OH)
6]3−
.
When heated with finely divided carbon or aluminium, it is reduced to chromium metal:
- Cr
2O
3 + 2 Al → 2 Cr + Al
2O
3
Unlike the classic thermite reaction involving iron oxides, the chromium oxide thermite creates few or no sparks, smoke or sound, but glows brightly. Because of the very high melting point of chromium, chromium thermite casting is impractical.
Heating with chlorine and carbon yields chromium(III) chloride and carbon monoxide:
- Cr
2O
3 + 3 Cl
2 + 3 C → 2 CrCl
3 + 3 CO
Chromates can be formed by the oxidation of chromium(III) oxide and another oxide in a basic environment:
- 2 Cr
2O
3 + 4 MO + 3 O
2 → 4 MCrO
4
See also
References
- "mp-19399: Cr2O3 (trigonal, R-3c, 167)". materialsproject.org. Retrieved 2019-12-20.
- NIOSH Pocket Guide to Chemical Hazards. "#0141". National Institute for Occupational Safety and Health (NIOSH).
- "Eskolaite". Webminerals. Retrieved 2009-06-06.
- J.E Greedan, (1994), Magnetic oxides in Encyclopedia of Inorganic chemistry R. Bruce King, Ed. John Wiley & Sons. ISBN 0-471-93620-0
- A. F. Holleman and E. Wiberg "Inorganic Chemistry" Academic Press, 2001, New York. ISBN 0-12-352651-5.
- Eastaugh, Nicholas; Chaplin, Tracey; Siddall, Ruth (2004). The pigment compendium: a dictionary of historical pigments. Butterworth-Heinemann. p. 391. ISBN 0-7506-5749-9.
- Gerd Anger, Jost Halstenberg, Klaus Hochgeschwender, Christoph Scherhag, Ulrich Korallus, Herbert Knopf, Peter Schmidt, Manfred Ohlinger, "Chromium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a07_067
- "Ammonium dichromate volcano". www.rsc.org. Retrieved 2019-02-26.
- R. Scholder "Sodium Hexahydroxochromate(III)" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 2, 1688ff.