Iron(III) nitrate
Iron(III) nitrate, or ferric nitrate, is the chemical compound with the formula Fe(NO3)3.
 | |
| Names | |
|---|---|
| IUPAC name
Iron(III) nitrate | |
| Other names
Ferric nitrate Nitric acid, iron(3+) salt | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.805 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Fe(NO3)3 | |
| Molar mass | 403.999 g/mol (nonahydrate) 241.86 g/mol (anhydrous) |
| Appearance | Pale violet crystals hygroscopic |
| Density | 1.68 g/cm3 (hexahydrate) 1.6429 g/cm3(nonahydrate) |
| Melting point | 47.2 °C (117.0 °F; 320.3 K) (nonahydrate) |
| Boiling point | 125 °C (257 °F; 398 K) (nonahydrate) |
| 150 g/100 mL (hexahydrate) | |
| Solubility | soluble in alcohol, acetone |
| +15,200.0·10−6 cm3/mol | |
| Structure | |
| octahedral | |
| Hazards[1] | |
| Safety data sheet | External SDS |
| GHS pictograms | 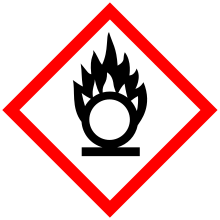  |
| GHS Signal word | Warning |
GHS hazard statements |
H272, H302, H319 |
| P210, P220, P221, P264, P270, P280, P301+312, P305+351+338, P330, P337+313, P370+378, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | non-flammable |
| NIOSH (US health exposure limits): | |
REL (Recommended) |
TWA 1 mg/m3[3] |
| Related compounds | |
Related compounds |
Iron(III) chloride Iron(III) sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydrates
Iron(III) nitrate is deliquescent, and it is commonly found as the nonahydrate Fe(NO3)3·9H2O, which forms colourless to pale violet crystals.
Other hydrates Fe(NO
3)
3·xH
2O, include:
- tetrahydrate (x=4), more precisely triaqua dinitratoiron(III) nitrate monohydrate, [Fe(NO
3)
2(H
2O)+
3][NO−
3]·H
2O, has complex cations where Fe3+
atom is coordinated with two nitrate anions as bidentate ligands and three of the four water molecules, in a pentagonal bipyramid configuration with two water molecules at the poles.[4]
- pentahydrate (x=5), more precisely penta-aqua nitratoiron(III) dinitrate, [Fe(NO
3)(H
2O)2+
5][NO−
3]
2, in which the Fe3+
atom is coordinated to five water molecules and a unidentate nitrate anion ligand in octahedral configuration.[4]
- hexahydrate (x=6), more precisely hexaaquairon(III) trinitrate, [Fe(H
2O)3+
6][NO−
3]
3, where the Fe3+
atom is coordinated to six water molecules in octahedral configuration.[4]
Chemical properties
Decomposition
When dissolved, iron(III) nitrate forms yellow solution due to hydrolysis. When heated to near boiling, nitric acid will evaporate from the solution, and all the iron will precipitate as iron(III) oxide Fe
2O
3.[5]
The compound will dissolve in molten stearic acid and decompose at about 120 °C to give iron(III) oxide-hydroxide FeO(OH).[6]
Preparation
The compound can be prepared by treating iron metal powder with nitric acid.
- Fe + 4 HNO3 → Fe(NO3)3 + NO + 2 H2O.
Applications
In the chemical laboratory
Ferric nitrate is the catalyst of choice for the synthesis of sodium amide from a solution of sodium in ammonia:[7]
- 2 NH3 + 2 Na → 2 NaNH2 + H2
Certain clays impregnated with ferric nitrate have been shown to be useful oxidants in organic synthesis. For example, ferric nitrate on Montmorillonite—a reagent called "Clayfen"—has been employed for the oxidation of alcohols to aldehydes and thiols to disulfides.[8]
Other applications
Ferric nitrate solutions are used by jewelers and metalsmiths to etch silver and silver alloys.
References
- HSNO Chemical Classification Information Database, New Zealand Environmental Risk Management Authority, retrieved 2010-09-19.
- "Iron(III) Nitrate Nonahydrate". American Elements. Retrieved June 20, 2019.
- NIOSH Pocket Guide to Chemical Hazards. "#0346". National Institute for Occupational Safety and Health (NIOSH).
- H. Schmidt, A. Asztalos, F. Bok and W. Voigt (2012): "New iron(III) nitrate hydrates: Fe(NO
3)
3·xH
2O with x = 4, 5 and 6". Acta Crystallographica Section C - Inorganic Compounds, volume C68, pages i29-i33. doi:10.1107/S0108270112015855 - Egon Matijević and Paul Scheiner (1978): "Ferric hydrous oxide sols: III. Preparation of uniform particles by hydrolysis of Fe(III)-chloride, -nitrate, and -perchlorate solutions". Journal of Colloid and Interface Science, volume 63, issue 3, pages 509-524. doi:10.1016/S0021-9797(78)80011-3
- Dan Li, Xiaohui Wang, Gang Xiong, Lude Lu, Xujie Yang and Xin Wang (1997): "A novel technique to prepare ultrafine Fe
2O
3 via hydrated iron(III) nitrate". Journal of Materials Science Letters volume 16, pages 493–495 doi:10.1023/A:1018528713566 - Hampton, K. G. Harris, T. M.; Hauser, C. R. (1973). "2,4-Nonanedione". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 5, p. 848 As of 2007, 22 other entries describe similar preparations in Organic Syntheses
- Cornélis, A. Laszlo, P.; Zettler, M. W. "Iron(III) Nitrate–K10 Montmorillonite Clay" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289.
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO 3)− 4 |
RONO2 | NO− 3 NH4NO3 |
HOONO2 | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)2 Fe(NO3)3 |
Co(NO3)2 Co(NO3)3 |
Ni(NO3)2 | CuNO3 Cu(NO3)2 |
Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru(NO3)3 | Rh(NO3)3 | Pd(NO3)2 Pd(NO3)4 |
AgNO3 Ag(NO3)2 |
Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt(NO3)2 Pt(NO3)4 |
Au(NO3)3 | Hg2(NO3)2 Hg(NO3)2 |
TlNO3 Tl(NO3)3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po(NO3)4 | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3 Ce(NO3)4 |
Pr(NO3)3 | Nd(NO3)3 | Pm(NO3)3 | Sm(NO3)3 | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy(NO3)3 | Ho(NO3)3 | Er(NO3)3 | Tm(NO3)3 | Yb(NO3)3 | Lu(NO3)3 | |||
| Ac(NO3)3 | Th(NO3)4 | PaO2(NO3)3 | UO2(NO3)2 | Np(NO3)4 | Pu(NO3)4 | Am(NO3)3 | Cm(NO3)3 | Bk | Cf | Es | Fm | Md | No | Lr | |||
