Strontium nitrate
Strontium nitrate is an inorganic compound composed of the elements strontium, nitrogen and oxygen with the formula Sr(NO3)2. This colorless solid is used as a red colorant and oxidizer in pyrotechnics.
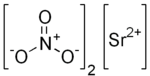 | |
| Names | |
|---|---|
| IUPAC name
Strontium nitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.107 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Sr(NO3)2 | |
| Molar mass | 211.630 g/mol (anhydrous) 283.69 g/mol (tetrahydrate) |
| Appearance | white crystalline solid |
| Density | 2.986 g/cm3 (anhydrous) 2.20 g/cm3 (tetrahydrate)[1] |
| Melting point | 570 °C (1,058 °F; 843 K) (anhydrous) 100 °C, decomposes (tetrahydrate) |
| Boiling point | 645 °C (1,193 °F; 918 K) decomposes |
| anhydrous: 710 g/L (18 °C) 660 g/L (20 °C) tetrahydrate: 604.3 g/L (0 °C) 2065 g/L (100 °C) | |
| Solubility | soluble in ammonia very slightly soluble in ethanol, acetone insoluble in nitric acid |
| −57.2·10−6 cm3/mol | |
| Structure | |
| cubic (anhydrous) monoclinic (tetrahydrate) | |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2750 mg/kg (rat, oral) |
| Related compounds | |
Other anions |
Strontium sulfate Strontium chloride |
Other cations |
Beryllium nitrate Magnesium nitrate Calcium nitrate Barium nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Strontium nitrate is typically generated by the reaction of nitric acid on strontium carbonate.[2]

Uses
Like many other strontium salts, strontium nitrate is used to produce a rich red flame in fireworks and road flares. The oxidizing properties of this salt are advantageous in such applications.[3]
Strontium nitrate can aid in eliminating and lessening skin irritations. When mixed with glycolic acid, strontium nitrate reduces the sensation of skin irritation significantly better than using glycolic acid alone.[4]
Biochemistry
As a divalent ion with an ionic radius similar to that of Ca2+ (1.13 Å and 0.99 Å respectively), Sr2+ ions resembles calcium's ability to traverse calcium-selective ion channels and trigger neurotransmitter release from nerve endings. It is thus used in electrophysiology experiments.
In popular culture
In his short story "A Germ Destroyer", Rudyard Kipling refers to strontium nitrate as the main ingredient of the titular fumigant.
References
- Patnaik, Pradyot (2002). Handbook of Inorganic Chemicals. McGraw-Hill, ISBN 0-07-049439-8
- Ward, R.; Osterheld, R. K.; Rosenstein, R. D. (1950). Strontium Sulfide and Selenide Phosphors. Inorganic Syntheses. 3. pp. 11–23. doi:10.1002/9780470132340.ch4. ISBN 978-0-470-13234-0.
- MacMillan, J. Paul; Park, Jai Won; Gerstenberg, Rolf; Wagner, Heinz; Köhler, Karl and Wallbrecht, Peter (2002) "Strontium and Strontium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a25_321
- Zhai H, Hannon W, Hahn GS, Pelosi A, Harper RA, Maibach HI (2000). "Strontium nitrate suppresses chemically-induced sensory irritation in humans". Contact Dermatitis. 42 (2): 98–100. doi:10.1034/j.1600-0536.2000.042002098.x. PMID 10703633.
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO 3)− 4 |
RONO2 | NO− 3 NH4NO3 |
HOONO2 | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)2 Fe(NO3)3 |
Co(NO3)2 Co(NO3)3 |
Ni(NO3)2 | CuNO3 Cu(NO3)2 |
Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru(NO3)3 | Rh(NO3)3 | Pd(NO3)2 Pd(NO3)4 |
AgNO3 Ag(NO3)2 |
Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt(NO3)2 Pt(NO3)4 |
Au(NO3)3 | Hg2(NO3)2 Hg(NO3)2 |
TlNO3 Tl(NO3)3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po(NO3)4 | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3 Ce(NO3)4 |
Pr(NO3)3 | Nd(NO3)3 | Pm(NO3)3 | Sm(NO3)3 | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy(NO3)3 | Ho(NO3)3 | Er(NO3)3 | Tm(NO3)3 | Yb(NO3)3 | Lu(NO3)3 | |||
| Ac(NO3)3 | Th(NO3)4 | PaO2(NO3)3 | UO2(NO3)2 | Np(NO3)4 | Pu(NO3)4 | Am(NO3)3 | Cm(NO3)3 | Bk | Cf | Es | Fm | Md | No | Lr | |||
