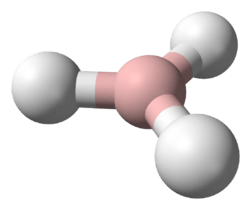
Semiheavy water
Semiheavy water is the result of replacing one of the protium in light water to deuterium.[1] It exists whenever there is water with light hydrogen (protium, 1H) and deuterium (D or 2H) in the mix. This is because hydrogen atoms (hydrogen-1 and deuterium) are rapidly exchanged between water molecules. Water containing 50% H and 50% D in its hydrogen contains about 50% HDO and 25% each of H2O and D2O, in dynamic equilibrium.[2] In regular water, about 1 molecule in 3,200 is HDO (one hydrogen in 6,400 is D). By comparison, heavy water D2O[3] occurs at a proportion of about 1 molecule in 41 million (i.e., one in 6,4002). This makes semiheavy water far more common than "normal" heavy water.
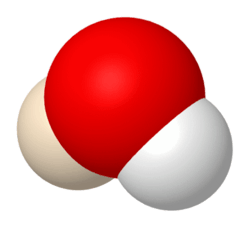 | |
| Names | |
|---|---|
| IUPAC name
(O-2H1)Water | |
| Other names
Deuterium hydrogen monoxide Deuterium hydrogen oxide, Water-d1 , Water-d | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| 115 | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| H2HO (also HDO) | |
| Molar mass | 19.0214 g mol−1 |
| Appearance | Very pale blue, transparent liquid, very similar to regular water |
| Density | 1.054 g cm−3 |
| Melting point | 3.81 °C (38.86 °F; 276.96 K) |
| Boiling point | 101.42 °C (214.56 °F; 374.57 K) |
| miscible | |
| log P | −0.65 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The freezing point of semiheavy water is close to the freezing point of heavy water. (3.8°C)
References
- Tashakor S (2016-09-28). "Neutronic Investigation of Semi-Heavy Water Application in Hplwr New Flow Pattern". CNL Nuclear Review: 1–5. doi:10.12943/CNR.2016.00019.
- Goncharuk VV, Kavitskaya AA, Romanyukina IY, Loboda OA (June 2013). "Revealing water's secrets: deuterium depleted water". Chemistry Central Journal. 7 (1): 103. doi:10.1186/1752-153X-7-103. PMC 3703265. PMID 23773696.
- "Heavy water | chemical compound". Encyclopedia Britannica. Retrieved 2019-04-24.
Further reading
- Schwarzer D, Lindner J, Vöhringer P (October 2005). "Energy relaxation versus spectral diffusion of the OH-stretching vibration of HOD in liquid-to-supercritical deuterated water". The Journal of Chemical Physics. 123 (16): 161105. doi:10.1063/1.2110087. hdl:11858/00-001M-0000-0012-E7B7-2. PMID 16268674.