Rubidium hydride
Rubidium hydride is the hydride of rubidium. It has the formula RbH and is an alkali metal hydride. It is synthesized using rubidium metal to react with hydrogen gas. As a hydride of an alkali metal, it is reactive towards even weak oxidizing agents. A redox reaction will occur with chlorine or fluorine and produce much heat. Rubidium hydride will react violently with water or air and careful storage is necessary.
 | |
| Names | |
|---|---|
| IUPAC name
Rubidium hydride | |
| Other names
Rubidium(I) hydride | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| RbH | |
| Molar mass | 86.476 g/mol |
| Appearance | white cubic crystals |
| Density | 2.60 g/cm3 |
| Melting point | Decomposes at 170°C |
| reacts | |
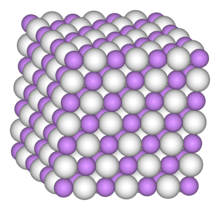
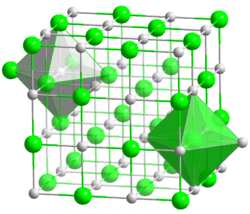
| Structure | |
| cubic, cF8 | |
| Fm3m, No. 225 | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-52.3 kJ/mol |
| Related compounds | |
Other anions |
Rubidium oxide Rubidium chloride |
Other cations |
Lithium hydride Sodium hydride Potassium hydride Caesium hydride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 4–79, ISBN 0-8493-0594-2
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.