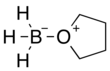
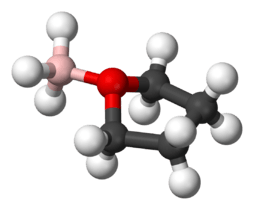
Borane–tetrahydrofuran
Borane–tetrahydrofuran is a dipolar bond charge-transfer complex composed of borane and tetrahydrofuran (THF). These solutions are used for reductions and hydroboration, reactions that are useful in synthesis of organic compounds.[1]
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) |
|||
| ECHA InfoCard | 100.034.424 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H11BO | |||
| Molar mass | 85.94 g·mol−1 | ||
| Appearance | White solid | ||
| Melting point | 66 °C (151 °F; 339 K) | ||
| Hazards | |||
| GHS pictograms |    | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H225, H260, H302, H315, H318, H319, H335 | ||
| P210, P223, P231+232, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+312, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P330, P332+313, P335+334, P337+313 | |||
| Flash point | −17 °C (1 °F; 256 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation and uses
The complex is commercially available but can also be generated by the dissolution of diborane in THF. A practical route to this is the oxidation of sodium borohydride with iodine in THF.[2]
The complex can reduce carboxylic acids to alcohols and is a common route for the reduction of amino acids to amino alcohols[3] (e.g. valinol). It adds across alkenes to give organoboron compounds that are useful intermediates.[4] The following organoboron reagents are prepared from borane-THF: 9-borabicyclo[3.3.1]nonane, Alpine borane, diisopinocampheylborane. It is also used as a source of borane (BH3) for the formation of adducts.[5]
Safety
The solution is highly sensitive to air, requiring the use of air-free techniques.[1]
References
- Marek Zaidlewicz, Herbert C. Brown, Santhosh F. Neelamkavil, "Borane–Tetrahydrofuran" Encyclopedia of Reagents for Organic Synthesis, 2008 John Wiley & Sons. doi:10.1002/047084289X.rb241.pub2
- Kanth, J. V. Bhaskar; Periasamy, Mariappan (1 September 1991). "Selective reduction of carboxylic acids into alcohols using sodium borohydride and iodine". The Journal of Organic Chemistry. 56 (20): 5964–5965. doi:10.1021/jo00020a052.
- McKennon, Marc J.; Meyers, A. I.; Drauz, Karlheinz; Schwarm, Michael (June 1993). "A convenient reduction of amino acids and their derivatives". The Journal of Organic Chemistry. 58 (13): 3568–3571. doi:10.1021/jo00065a020.
- Kabalka, George W.; Maddox, John T.; Shoup, Timothy; Bowers, Karla R. (1996). "A Simple And Convenient Method For The Oxidation Of Organoboranes Using Sodium Perborate: (+)-isopinocampheol". Organic Syntheses. 73: 116.
- Crépy, Karen V. L.; Imamoto, Tsuneo (2005). "Preparation Of (S,S)-1,2-Bis-(tert-butylmethylphosphino)ethane ((S,S)-t-Bu-BISP*) As A Rhodium Complex". Organic Syntheses. 82: 22.